ABSTRACT The differences in satellite DNA
methylation pattern of corn seedlings with various spontaneous chromosome
aberration yields and changes in methylation pattern of these DNA sequences
under different exposure modes of acute UV-C and chronic gamma-irradiations
have been investigated. The obtained experimental data and the conducted
correlation analysis demonstrated the significant correlation between the
satellite DNA methylation pattern varieties and chromosome aberration yields
under various stress exposure modes. The role of satellite DNA methylation
pattern variability and its changing in key responses to stress such as mobile
elements’ activation, cell’s passage of checkpoints, and homological repair was
discussed. Keywords: Stress Response; Plant Resistance; Satellite DNA
Methylation Pattern; Brave-Pirson Linear Correlation 1. Introduction Changing
organism’s resistance to stress factors, various reactions, which role in this
process depends on factor’s acting rate, duration and/or periodicity.
Complexity of interactions in stress reactions can also be attributed to
hierarchical-structural and functional, organism organization, where different
processes have various sensitivities and times of development. DNA methylation
is one of the most important and polyfunctional mechanisms of biological
regulation, which has a great significance in such epigenetic processes as
genomic imprinting, differentiation, apoptosis and morphogenesis, aging of an
organism, regulation of mobile elements’ activity [1-3]. It is also known that
methylation of cytosine is the natural factor of mutagenesis [2] and at the same
time it is a factor affecting regional DNA structure’s organization that is
necessary for successful passage of enzymatic reactions, related to reading-out
of information and reparation. Plants contain most of methylated cytosine (up
to 30%); the DNA methylation of these organisms is the result of functioning
four groups of methyltransferases [2] that provide a great methylation sites’
variety. It may be claimed that various methylation pathways can play important
roles in stress response reactions and rearrangements of their resistance,
whereas in alternative “to run or to fight” these organisms choose “fighting”
at all levels of organization. A number of last investigations show some
changes in level and pattern of DNA methylation under biotic [3,4] and different
forms of abiotic stress—dryness [3], salinization [3-6], radiation exposures
with various dose rates [7-9] and duration [8,10]. Polyfunctional of DNA
methylation process also allows different ways of its participation both in
failure (e.g. activization of mobile elements, initialization of genome
instability) and/or formation of active protective reactions, associated with
metabolism reorganization. Thus changes in DNA methylation level and/or pattern
under different stress exposure [3-10] still require specification of their
biological significance. The appearance of DNA micro array technology made a
revolution in studying changes in gene expression under stress exposures.
Obtained data have confirmed the connection between changes in methylation pattern
of transcribed DNA with changes in expression of major gene groups, metabolism
rearrangements and resistance changes under stress exposure [11-13]. Copyright
© 2013 SciRes. CellBio 164 D. A. SOKOLOVA ET AL. In parallel with studying the
majority of changes in transcribed DNA methylation pattern in their
responsiveness under stress exposures, great changes in satellite DNA
methylation pattern have been detected [14]. It is known that satellite DNA is
true to type component of eukaryotic genome. It consists of tandem organized
repeats, and it is never transcribed or encoded proteins and is located in
heterochromatin part of chromosome [15]. A high methylation level of satellite
DNA’ cytosine has been shown but satellite DNA’s biological importance still
hasn’t been understood. A question about biological role of changes in DNA
methylation pattern under stress exposures and subsequent changes of cell
resistance is also unexplored now. The paper is dedicated to investigate the
connection between variability of satellite DNA methylation pattern and
spontaneous chromosome aberration’ rate as well as changes in methylation
pattern of satellite DNA under different modes of acute UV-C and chronic
gamma-exposure of seedlings. The study of DNA methylation pattern is performed
by comparing the chromosomal aberrations yielded in meristematic tissues as the
independent index that allowed to estimate the plant cell resistance. 2.
Material and Method The investigation of connection between satellite DNA
methylation statuses with plant cell resistance to stress exposure was carried
out in three series of experiments: 1) Acute UV-C exposure of epigenetically
different corn seedlings (EDS). Preliminary three groups of corn’ seedlings
with different germination rates were empiric selected: fast germinating (F-G),
middle germinating (MG), and slowly germinating (S-G). A great connection
between germination rates and differences in transcribed DNA methylation
pattern has been preinstalled; 2) Acute UV-C exposure in the mode of “adaptive
exposure-challenge exposure” with different ranges between the adaptive UV-C
irradiation and challenge one (different mode UV-C exposure). The adaptive dose
was 1 kJ/m2 and the challenge one—6.2 kJ/m2 ; Combined exposure: preliminary
chronic gamma-exposure of dry seeds with various accumulative dose and
subsequent seedlings acute UV-C exposure. Two intervals between the adaptive
UV-C irradiation and challenge one were investigated: 4 hours and 24 hours. The
necessity to expose seedlings in challenge dose (6.2 kJ/m2 ) and whole dose
(7.2 kJ/m2 ) in the same physiological state was taken into account. Thus such
variants of irradiation were used: 1) Non UV-C irradiated seedlings; 2)
Adaptive exposure (1 kJ/m2 ); 3) Adaptive exposure, in 4 hours-challenging one
(6.2 kJ/m2 ); 4) Whole dose exposure (7.2 kJ/m2 ); exposure simultaneously with
the challenging irradiation of variant 3; 5) Adaptive exposure, in 24
hours—challenging one (6.2 kJ/m2 ); 6) Whole dose exposure (7.2 kJ/m2 );
irradiation simultaneously with the challenging irradiation of variant 5. Such
ways of irradiation were conducted both with seedlings from non preliminary
gamma-irradiated seeds (NPI) and with seedlings from preliminary
gamma-irradiated seeds (PI). The study was performed using 3 - 7-days maize
seedlings, sort Titan. Seeds’ sprouting was conducted on bottom plates with wet
filter paper, in thermostat under the temperature +23˚C - +24˚C. Bactericidal
irradiator of the open type OBN-150М (Ukraine) with Philips Special TUV 30 W lamps
was used. Three-day seedlings were exposed by UV-C in whole doses of 7.2 kJ/m2
(dose rate was 6.2 W/m2 ) in the range 4 hours and 24 hours between adaptive
and challenging irradiation as described above. A glass container with 137CsCl2
was used for investigation of chronic exposure effects; dry seeds were exposed
with dose rate 30 mR/h, accumulated dose reached 3.5 Gy. The apical root
meristems were used as an object for cytogenetic analysis. Sampling was carried
out on the 4th day after irradiation. Detached apexes have been put to the
Brodsky’ fixative (acetic acid: ethanol: formalin = 0.3:1:3) for two hours with
following washing by 70% ethanol (3 - 4 times). Maceration has been performed
by alkaline hydrolysis with 20% NaOH over two hours. Then preparations have
been washed in distilled water for 15 minutes. Staining was carried out by
acetoarsein and hydrochloric acid mixture (acetoarsein: 1M HCl = 1:1) over 16 -
18 hours. Stained samples have been washed in 45% CH3COOH with following
preparation the crushed specimens. Ten alternative apexes were used and 5 - 10
thousands of cells were analyzed for every variant. The unstable chromosomal
aberrations were detected using anaphase-telophase technique due to plant
tissue specificity. In spite of this cells’ sampling has averaged over 300 -
350 chromosomal aberrations during the anaphase in each preparation. A
cytogenetic analysis was conducted on the light microscope “Jenaval” (Germany).
Independent cytogenetic analisis was performed 8 times. Significance level (α)
of assessment is 0.05. Isolation of DNA was performed from the 6-day-old corn
seedlings with the set of reagents DiatomTM DNA Prep100 based on
NucleoS-sorbent. The standard protocol for DNA extraction provided by the
manufacturer was used. The concentration of DNA solution was measured by
BioPhotometer Plus Eppendorf v.1.35 using standard technique [16,17]. The PCR
was carried out in the four-channel DNACopyright © 2013 SciRes. CellBio D. A.
SOKOLOVA ET AL. 165 amplifier “Tercik” (“DNA-Technology”, Moscow). One primer
has been used: inter simple sequence repeatISSR (15-soro,
sequence-5’-АС-АС-АС-АС-АС-АС-АС- АС--3’), were synthesized by company
“Metabion” (Germany) [18]. The restriction analysis as well as the PCR was
carried out in the four-channel DNA-amplifier “Tercik” (“DNATechnology”,
Moscow). Two types of restriction enzymes-isoschizomers were used: HpaII (5’.C
CGG.3’), MspI (5’.C CGG.3’) and restrictase MboI (“Fermentas”, Germany).
Reactions were performed according to the conventional manual by the supplier
(Table 1). The reaction mixture for the HpaII-analysis (total volume 25 μl)
contained: 0.2 μl HpaII, 2.0 μl 10хBuffer Tango, 1.5 μg total DNA and 17.7 μl
deionized water. The mixture has been covered with the 20 μl of mineral oil.
The reaction mixture for the MspI-analysis (total volume 25 μl) contained: 0.6
μl MspI, 2.0 μl 10хBuffer Tango, 1.5 μg total DNA and 17.1 μl deionized water.
The mixture has been covered with the 20 μl of mineral oil. The reaction
mixture for the MboI-analysis (total volume 25 μl) contained: 0.2μl MboI, 2.0
μl 10хBuffer Tango, 1.5 μg total DNA and 17.7 μl deionized water. The mixture
has been covered with the 20 μl of mineral oil. The conditions for restriction
reactions were: 16 hours under 37˚C, then 20 min under 65˚C (for HpaII and
MboI) and 20 min under 80˚C (for MspI) to stop the reactions. Products of PCR
and restriction analysis were separated in 1.0% agarose gel with TBE-buffer at
the presence of ethidium bromide, and visualized in UV-transilluminator. The same
volume of PCR and restriction products (10 μl) was brought into the gel
pockets. The FastRuler High Range DNA Ladder (“Fermentas”, Germany) with
fragments’ length 10,000, 4000, 2000, 1000 and 500 base pairs and the FastRuler
Low Range DNA Ladder (“Fermentas”, Germany) with fragment length 1500, 850,
400, 200 and 50 base pairs were used as a molecular weight markers. Independent
ISSR-PCR was performed 8 times also. Experimental findings statistical
analysis–the variance value and the Brave-Pirson’s correlation coefficientwere
calculated with traditional method [19]. Table 1. Restriction enzymes and their
sites of recognition/restriction. Restriction enzyme Sites of
recognition/restriction MspI HpaII MboI 5’C…C*CG, C…5’ 5’…C*CGG…3’ 3’G…G C*C…5’
5’…C*CGC…3’ 3’…CT…AG *C…5’ 3. Results and Discussion The obtained cytogenetic
data pointed out major varieties in chromosome aberrations’ yield (Ab, %)
appeared among groups F-G, M-G and S-G seedling (Figure 1). The
electrophoregram of isolated DNA nativity is shown in Figure 2. The
electrophoregram of native DNA amplification with ISSR primers (Figure 3) shows
specific differences Figure 1. The chromosome aberration yield (α = 0.05) in
root meristem of corn seedlings with various germination rates;
C-non–irradiated seedlings; UV-C-seedlings irradiated with UV-C. M 1 2 3 4 5 6
Figure 2. The electrophoregram of isolated DNA quality. М—high-molecular-weight
marker; 1—“FG” sample; 2— “FG + UV-C” sample; 3—“MG” sample; 4—“MG + UV-C”
sample; 5—“SG” sample; 6—“SG + UV-C” sample. M 1 2 3 4 5 6 Figure 3. The
electrophoregram of native DNA amplification products with ISSR primers.
М—high-molecular-weight marker; 1—“FG” sample; 2—“FG+UV-C” sample; 3— “M-G”
sample; 4—“M-G + UV-C” sample; 5—“SG” sample; 6—“S-G + UV-C” sample. Copyright
© 2013 SciRes. CellBio 166 D. A. SOKOLOVA ET AL. in amplicons range of
irradiated and unirradiated fastgrowing seedlings (positions 1 and 2). These
data do not contradict with data about good nativity of isolated DNA. The most
appropriate explanation is connected with appearance of damage during PCR that
might indirectly evidence about low methylation level of this DNA part in
fast-growing seedlings resulting to greater vulnerability of these DNA samplers
1. An electrophoregram of the amplification products obtained by ISSR-PCR of
the MspI restriction products (Figure 4) illustrated the differences in DNA
methylation pattern among seedlings with various germination rates (positions
1, 3, 5). The electrophoregram of fast-germinated seedlings (FG, position 1)
had four distinct groups of amplicons with almost the same number of DNA
fragments. The groups of amplicons (positions 3 and 5) for variants “M-G” and
“S-G” had the same molecular weight, but different number of DNA fragments. The
comparison of positions 1 and 2, 3 and 4, 5 and 6 of this electrophoregram
(Figure 4) shows great changes of satellite DNA methylation pattern after
irradiation. Positions 2, 4, 6 are also differing from each others that
correspond to increased chromosome aberration’ yield after UV-C exposure
(Figure 1). Also considerable differences between methylation patterns of
satellite DNA of seedlings that initially had various germination rates
(positions 1, 3, 5) were observed in separating amplification products of MboI
restricts with ISSR–primers (Figure 5). There was just one type of amplicons
for “F-G” seedlings and great differences between “M-G” and “S-G” variants.
Electrophoregram for “M-G” seedlings had four distinct groups of amplicons with
comparatively more high-molecular fragments. The comparison of positions 1 and
2, 3 and 4, 5 and 6 of this electrophoregram (Figure 5) shows great changes of
satellite DNA methylation pattern after irradiation. M 1 2 3 4 5 6 Figure 4.
The electrophoregram of the amplification products obtained by ISSR-PCR of the
MspI restriction products. М—high-molecular-weight marker; 1—“FG” sample; 2—“FG
+ UV-C” sample; 3—“MG” sample; 4—“MG + UV-C” sample; 5—“SG” sample; 6—“SG +
UV-C” sample. M 1 2 3 4 5 6 Figure 5. The electrophoregram of the amplification
products obtained by ISSR-PCR of the MboI restriction products.
М—high-molecular-weight marker; 1—“FG” sample; 2—“FG + UV-C” sample; 3—“MG”
sample; 4—“MG + UV-C” sample; 5—“SG” sample; 6—“SG + UV-C” sample. Positions 2,
4 do not have major differences from each other. The greatest difference is
observed between positions 4 and 6. Such differences correspond to various
increasings in the chromosome aberration’ yield after UVC exposure (Figure 1).
Thus original difference in satellite DNA methylation pattern is connected to
differences in pattern changes under irradiation exposure and chromosome
aberration’ yield. This indicates both different effectiveness of repair
processes or various original sensitiveness to damage. Consider the data about
acute UV-C exposure mode “adaptive-challenging irradiation” as well as combined
exposure whereby seedlings growing from preliminary gamma-irradiated seeds have
been exposed. Chromosome aberration yield in root meristematic tissue (Figure
6) indicates to major differences in appearance of seedlings’ adaptive
reactions that have grown from unexposed and gamma-exposed seeds. Chronic
radiation exposure of seeds causes increase of chromosome aberration rate in
seedlings’ root meristematic tissues. UV-C exposure of seedlings from
preliminary unirradiated seeds with adaptive dose leads to increasing
chromosome aberration yield whereas exposure of seedlings from preliminary
irradiated seeds causes the hormetic effect. Exposure mode “adaptive, in 4
hours-challenging” causes the appearance of adaptive response for seedlings
without preliminary irradiation exposure; with interval in 24 hours between
adaptive and challenging exposure the adaptive response haven’t been observed.
Seedlings from preliminary irradiated seeds didn’t show the adaptive response
with both intervals between adaptive and challenging irradiation. An
explanation of such phenomena from the standpoint about meristematic tissue’
heterogeneity and possibility of two forms of repopulation renewal is given in
paper [14]. The object of this study is to compare stability changes to stress
factor affecting and changes in satCopyright © 2013 SciRes. CellBio D. A.
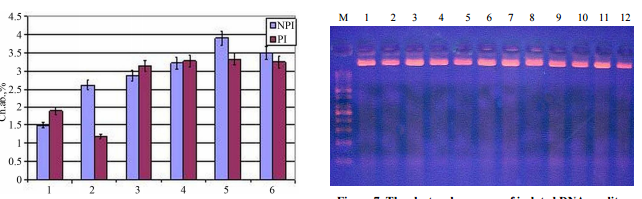
SOKOLOVA ET AL. 167 Figure 6. The chromosome aberration yield (α = 0.05) in
root meristem of corn seedlings from non preliminary gamma-irradiated seeds
(NPI), and preliminary gammairradiated seeds, ( PI ) with UV-C irradiation mode
as described in “ Material and Methods”. ellite DNA methylation pattern. The
electrophoregrams of DNA quality checking are shown in Figure 7. These results
demonstrate the absence of meaningful DNA fragmentation that would take a place
in apoptosis inducing by UV-C irradiation. The electrophoregram of native DNA
amplification shows specific differences in comparison with all other variants
of amplicon’s range for variant “adaptive-challenge exposure in 24 hours”
(Position 6). The most appropriate explanation is connected to appearance of
ulterior (single-stranded) injuries during PCR. It’s essential that the
features of DNA fragmentation are observed in a variant exposed with full dose
at a time. For Figures 7-11: 1. Total control (NPI and non UV-C irradiated
seedlings); 2. NPI + adaptive exposure; 3. NPI + adaptive exposure, in 4
hours-challenging one; 4. NPI + whole dose exposure (7.2 kJ/m2 ); exposure
simultaneously with the challenging irradiation of variant 3); 5. NPI +
adaptive exposure, in 24 hours-challenging one; 6. NPI+ whole dose exposure;
irradiation simultaneously with the challenging irradiation of variant 5; 7. PI
+ non UV-C irradiation; 8. PI + adaptive exposure; 9. PI + adaptive exposure,
in 4 hours–challenging one;10.PI + whole dose exposure; exposure simultaneously
with the challenging irradiation of variant 3 and 9); 11. PI + adaptive
exposure, in 24 hours-challenging one; 12. PI + whole dose exposure;
irradiation simultaneously with the challenging irradiation of variants 5 and
11. The electrophoregram of MboI restricts’ ISSR amplification shows various
differences in DNA methylation patterns according to exposure mode. Comparison
of positions 1 (seedlings from seeds without preliminary irradiation) and 7
(seedlings from preliminary gammairradiated seeds) indicates to major
differences in range of amplicons: as a result of dry seeds chronic exposure
the satellite DNA methylation pattern of seedlings shows some complication on
electrophoregram because of apM 1 2 3 4 5 6 7 8 9 10 11 12 Figure 7. The
electrophoregram of isolated DNA quality. M 1 2 3 4 5 6 7 8 9 10 11 12 Figure
8. The electrophoregram of native DNA ISSR-amplification. M 1 2 3 4 5 6 7 8 9
10 11 12 Figure 9. The electrophoregram of MboI restricts’ ISSR amplification.
M 1 2 3 4 5 6 7 8 9 10 11 12 Figure 10. The electrophoregram of
ISSR-amplification of MspI restricts. Copyright © 2013 SciRes. CellBio 168 D.
A. SOKOLOVA ET AL. M 1 2 3 4 5 6 7 8 9 10 11 12 Figure 11. The electrophoregram
of HpaII-restricts ISSRamplification. pearance of amplicons with low and middle
weight. It’s essential that variants 9 - 12 show identical ranges and parallels
with the same chromosome aberration yield in the variants are also possible
(Figure 6). The electrophoregram of ISSR-amplification of MspI restricts shows
various changes in DNA methylation pattern according to exposure mode.
Comparison of positions 1 (seedlings from seeds without preliminary
irradiation) and 7 (seedlings from preliminary gamma-exposed seeds) indicates
to major differences in amplicons range: after dry seeds’ chronic irradiation
satellite DNA methylation pattern leads to great complication of
electrophoregram because of appearing low-weight amplicons indicated to
increase of restriction MspI sites. Major difference of 8th variant is
observed, which demonstrates hormesis effect in terms of chromosome aberration
yield. It’s essential that variants 9 - 12 show identical ranges as well as
MboI enzyme. Electrophoregram of HpaII-restricts’ ISSR-amplification indicates
to less dependence of amplicon range from exposure mode. The differences
between positions 1 (seedlings from seeds without preliminary irradiation) and
7 (seedlings from preliminary gamma-irradiated seeds) are also visible because
of less content of highmolecular weight fragments. Great difference of variants
9 and 10-12 that corresponds almost identical chromosome aberration yield is
observed (Figure 6). Quantify connection between changes in satellite DNA
methylation patterns and chromosome aberrations’ yield under various affects
using Brave-Pirson’s linear correlation. To perform such approach we have to
suggest some principals of quantifying various changes on electrophoregrams and
their degrees. There are several significant quantitative characteristics of
DNA methylation pattern changes that could be registered on electrophoregrams:
1) Change of general amplicons’ number; wherein following versions are possible:
a) Changes in molecular mass of amplicons, i.e. position related to ladder
bands on electrophoregram but within the amplicons’ mass of control variant;
nevertheless the number of new control bands or their disappearance could be
various; b) The appearance of amplicons with mass that greatly exceed the
limits of control bands’ mass both in the range of more high molecular mass
and; 2) Change of bands’ brightness–that indicates to changing number of
amplicone’ fragments of the same mass; 3) The combination of the listed above
quantitative indicators. Interactions between these various indicators greatly
exceed the classification possibilities of changes in DNA methylation pattern
and accordingly the correlation estimation between their changing rates. Consider
the simplest connection type-linear correlation between the number of amplicons
and the chromosome aberrations’ yield for various series of experiments. The
statistical analysis for each experimental series was conducted separately.
Correlation indexes shown in Table 2 indicate to existence of significant (α =
0.05) positive correlation between amplicons’ number and chromosome aberration
yield for experiments with acute UV-C exposure and MboI enzyme and significant
negative correlation (α= 0.01) just for experiments with composed radiation
exposure and MspI ans MboI enzymes. To continue the correlation analysis using
more detail approach via determination of 5 grades (from 0 to 4) of methylation
patterns’ varieties. It will be used following indexes: 0—The absence of
differences according to control variant; 1—The differences in amplicons’
number, which mass is in the range of control amplicons’ mass; 2—The
differences in amplicons’ number, which mass is in the range of control
amplicons’ mass + differences in brightness of bands that indicates to various
number of fragments in one amplicon; 3—The differences in amplicons’ number,
which mass is not in the range of control amplicons’ mass; 4—The differences in
amplicons’ number, which mass is not in the range of control amplicons’ mass +
differTable 2. The coefficient of correlation between amplicons’ number and
chromosome aberration yield. Correlation Coefficient, R Experimental series
MspI HpaII MboI 1. 0.29 - 0.72 2 0.69 0.105 0.82* 3 −0.89** −0.73 −0.89**
Significance of a correlation coefficient, * α = 0.05, **α = 0.01. Copyright ©
2013 SciRes. CellBio D. A. SOKOLOVA ET AL. 169 ences in brightness of bands.
Results of this way of correlation assessment are shown in Table 3. Thus such
approach for determination the degree of methylation pattern changes increased
the correlation index for some variants and decreased it for another one.
Continue the specification of approach to correlation assessment via
determination of 9 grades (from 0 to 8) of methylation patterns’ varieties. It
will be used following indexes: 0—The absence of differences according to
control variant; 1—The differences in amplicons’ number (n), which mass is in
the range of control amplicons’ mass, n ≤ 3; 2—The differences in amplicons’
number (n), which mass is in the range of control amplicons’ mass, n ≤ 3 +
differences in their brightness; 3—The differences in amplicons’ number (n >
3), which mass is in the range of control amplicons’ mass; 4—The differences in
amplicons’ number (n > 3), which mass is in the range of control amplicons’
mass + differences in their brightness; 5—The differences in amplicons’ number
(n ≤ 3), which mass is not in the range of control amplicons’ mass; 6—The
differences in amplicons’ number (n ≤ 3), which mass is not in the range of
control amplicons’ mass + differences in their brightness; 7—The differences in
amplicons’ number (n > 3), which mass is not in the range of control
amplicons’ mass; 8—The differences in amplicons’ number (n > 3), which mass
is not in the range of control amplicons’ mass + differences in their
brightness. Results of this way of correlation assessment are shown in Table 4.
Specification of differences between electrophoregrams and number of their
grades could be continued using additional characteristics of electrophoregrams
and their combination. However performed correlation analysis using three
approaches allows to make general Table 3. The coefficient of correlation
between 5 grades of electrophoregram varieties and chromosome aberration yield.
Correlation Coefficient, R Experimental series MspI HpaII MboI 1 0.27 - 0.57 2
0.81* 0.77 0.91** 3 0.43 0.43 0.64 * α = 0.05; **α = 0.01. Table 4. The
coefficient of correlation between 9 grades of electrophoregram varieties and
chromosome aberration yield. Correlation Coefficient, R Experimental series
MspI HpaII MboI 1 0.57 - 0.64 2 0.87* 0.84* 0.89** 3 0.21 0.71 0.7 * α = 0.05;
**α = 0.01. conclusion about existence of quantitative connection between
chromosome aberration yield like both integral cell stress response and changes
in satellite DNA methylation pattern. Performed analysis also show that unique
approach to quantify connection between chromosome aberration yield and their
rates on electrophoregrams doesn’t exist. Such suggestion points to possible
difference in mechanisms of cell response to exposure type (physical exposure,
exposure rate and duration and so on). The investigation of differential gene
activity using micro array methods and changes of DNA methylation pattern indicated
that according to exposure type and intensity the activity of various gene
groups had changed. That’s for satellite DNA–its direct or indirect
participation in cell stress response could be related to different mechanisms
according to exposure type. Despite of ways of satellite DNA participation in
stress reaction such mechanisms are different as well as for transcribed DNA.
It should have been emphasized that for experimental series 1 with 3
polymorphic groups of plants significant correlations weren’t obtained with any
criteria. Reason for such phenomena is connected to original epigenetical
polymorphism of biological material and deficient sampling from 6 variants for
correlation assay. 4. Conclusions Comparison of the results of cytogenetic
analysis with changes in methylation patterns of satellite DNA after
irradiation pointed out to their connections with different stress tolerance.
Change of the satellite DNA methylation profile may reflect the mobile elements
activization, mostly associated with satellite DNA [13], and indicate the
damage’s progress. Such ability is especially essential for corn; it’s known
that nearly 50% satellite DNA of the plant are represented with mobile elements
[2,3,15]. At the same time, it can result in DNA configuration changes and has
the protective effect. Since functional importance of satellite DNA was
explained in part by Copyright © 2013 SciRes. CellBio 170 D. A. SOKOLOVA ET AL.
conceptions, it was assumed to have a structural role in spatial organization
of genome, and take part in homologous chromosomes’ conjugation during meiosis
and replication of chromosomes’ telomeric sites [15]. Probably in this case
different methylation patterns of satellite DNA, which meant various chromatin
conformations, could have interactive character: specific methylation patterns
of transcribed DNA may play role in transcription processes only under definite
conformation of all the chromatin. Interaction between satellite DNA
methylation pattern and resistance to external exposures might have another
explanation. It could result not only from efficient functioning of repair
systems of spontaneous и inducible DNA injuries, but also from systems
responsible for passing cell cycle checkpoints and complete repair of
double-stranded DNA breaks. It was known, that effective repair of
double-stranded DNA breaks with the mechanism of homologous recombination was
possible only under conditions of certain level of chromatin relaxation [20],
so it was also associated directly to the conformation of satellite DNA. Thus
conducted research provides grounds to suggest that satellite DNA methylation
patterns and their changes might have various roles in cell response to stress
factor. All the functions are mediated by conformation changes of these DNA sequences.
5. Acknowledgements Funding for the study was provided by the Academy of
Science of Ukraine, Grant No. III-3-08 “Epigenetic components of plant
adaptation”. We thank PhD, Head of Laboratory of Molecular Genetics Morgun
B.V., Institute of Cell Biology and Genetic Engineering, National Academy of
Science of Ukraine for help in method mastering.