ABSTRACT Oxidative damage and redox metal
homeostasis loss are two contributing factors in brain aging and widely
distributed neurodegenerative diseases. Oxidative species in company with
excessive amounts of intracellular free iron result in Fenton-type reaction
with subsequent production of highly reactive hydroxyl radicals which initiate
peroxidation of biomolecules and further formation of non-degradable toxic
pigments called lipofuscin that amasses in long-lived postmitotic cells such as
neurons. Dietary flavonoid baicalein can counteract the detrimental
consequences through exertion of a multiplicity of protective actions within
the brain including direct ROS scavenging activity and iron chelation. In this
study, we evaluated the neuroprotective effects of baicalein in menadione
(superoxide radical generator)-treated SK-N-MC neuroblastoma cell line. Our
results showed that treatment of cells with menadione led to lipofuscin
formation due to elevated intracellular iron contents and accumulation of
oxidative products such as MDA and PCO. Also, menadione caused apoptotic cell
death in SK-N-MC cells. However, pretreatment with baicalein (40 μM) reversed
the harmful effects by chelating free iron and preventing biomolecules
peroxidations. Moreover, baicalein prevented cell death through modulation of
key molecules in apoptotic pathways including suppression of Bax and caspase-9
activities and induction of bcl2 expression. Key structural features such as
presence of hydroxyl groups and iron-binding motifs in baicalein make it the
appropriate candidate in antioxidant-based therapy in age-related
neurodegenerative diseases. Keywords: Aging; Baicalein; Lipofuscin; Menadione;
Neurodegenerative Disease; Oxidative Stress 1. Introduction The key precept of
the oxidative stress theory of aging is that senescence-related loss of
function is due to the progressive and irreparable accrual of molecular
oxidative damage which is brought about by powerful pro-oxidant species
including reactive oxygen species (ROS) [1,2]. ROS include a broad range of
partially reduced metabolites of oxygen (e.g. superoxide, hydrogen peroxide and
hydroxyl radical) having higher reactivity than molecular oxygen [3]. Their
raison d’être remains unclear. Putative explanations for their occurrence range
from inadvertent by-products of aerobic metabolism to highly regulated and
intricate signaling mechanisms [4]. Free radical or oxidative stress theory of
aging was first proclaimed by Denham Harman demonstrating the role of oxidative
species in aging process acceleration and cell death [5,6]. This theory can
explain many of the senescent changes including accumulation of brown-yellow,
electron-dense, autofluorescent bodies in cells called lipofuscin pigments or
age pigments [5,7,8]. Correlation of lipofuscin with aging is not only because
the amount of lipofuscin elevates with age, but also, more significantly
because the rate of lipofuscin accumulation negatively correlates to longevity.
High consumption of oxygen via brain makes it susceptible to oxidative damage
[9,10]. Reactive oxygen species which are generated by mitochondria through
different ways, diffuse into lysosomes which encompass a variety of
macromolecules under degradation as well as redox-active low molecular iron
which would be released from different sorts of metalloproteins. Based on
Fenton reaction, hydroxyl radical can be generated through the reaction of
hydrogen peroxide with iron, bringing about the cross-linking of adjacent
macromolecules and resultant lipofuscin formation [7,8,10-12]. There is a
debate on the function of lipofuscin pigments formed during exposure of cells
to oxidative agents. Some researchers believe that lipofuscin formation does
not have any serious effects on normal func- * Corresponding author. Copyright
© 2013 SciRes. CellBio 36 M. MOSLEHI, R. YAZDANPARAST tion of cells. On the contrary,
some scientists state that although lipofuscin cannot react directly with
extralysosomal constituents because of the lysosomal membrane, the high content
of iron within lipofuscin granules may promote generation of ROS, sensitizing
cells to oxidative injury through lysosomal destabilization. Destabilization of
lysosomal membrane results in leaking of hydrolytic enzymes into the cytosol.
Hence, oxidative species and redox-active transition metals homeostasis
impairments which facilitate further formation of active and hazardous reactive
oxygen species might be two main characteristics of age-related
neurodegenerative diseases [13]. Human’s aspiration for greater longevity has
long been a strong motivation for a lot of studies in the field of aging and age-related
disorders. Escalating body of evidence implies that lifestyle factors, and
specially the diet, may counteract oxidative damage [2,14]. Dietary flavonoids
with blood-brain barrier ability were shown to have potential anti-aging and
brain-protective activities [5, 15-18]. Baicalein
(5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one), one of the naturally
occurring flavonoids in Scutellaria baicalensis GEORGI known as “Huang qin” in
China and “Ogon” in Japan, is prescribed for oxidative stress-related diseases
[19]. Numerous studies have shown that baicalein protects neurons from
oxidative damage via multiple bio-effects ranging from classic radical
scavenging activities to modulation of signaling pathways involved in
stress-associated diseases. Moreover, recent studies have denoted that
baicalein mitigates formation of hydroxyl radical through its iron-binding
(anti-Fenton) and strong chelation properties [20-23]. In this study, we
scrutinize the effect of baicalein on menadione (superoxide anion generator)-induced
lipofuscin formation in human neuroblastoma SK-N-MC cell line to comprehend the
mechanism by which baicalein protect SK-N-MC cells against oxidative damages.
2. Materials and Methods 2.1. Materials The cell culture medium (RPMI-1640),
penicillin-streptomycin and fetal bovine serum (FBS) were purchased from Gibco
BRL (Life technology, Paisely, Scotland). The culture plates were purchased
from Nunc (Brand products, Denmark). dimethyl sulfoxide (DMSO), FeCl3 and KMnO4
were obtained from Merck (Darmstadt, Germany). Ethidium bromide, acridine
orange, Baicalein and Triton X-100 were purchased from Pharmacia LKB
Biotechnology (Sweden). MTT [3-(4,5-dimethyl tiazol-2, 5-diphenyl tetrazolium
bromide], phenylmethylsulphonyl fluoride (PMSF), leupeptin, pepstatin,
aprotinin, monochlorobimane (mBCL), dithionitrobenzoic acid (DTNB), GSH,
ascorbic acid, ferrozine and pan-caspase inhibitor (Z-VAD-fmk) were purchased
from Sigma Chem. Co. (Germany). 2’,7’-dichlorofluorescein diacetate (DCFHDA)
was obtained from Molecular Probe (Eugene, Oregon, USA).
Ethylenediaminetetraacetic acid (EDTA) was from Aldrich (Germany). Human SK-N-
MC neuroblastoma cells were obtained from Pasteur Institute (Tehran, Iran). All
antibodies including anti-Bax, anti-Bcl-2, anticleaved caspase-9, anti-tubulin
and mouse/rabbit horseradish peroxidase-conjugated second-dary antibodies were
purchased from Biosource (Nivelles, Belgium). Chemiluminescence detection
system was purchased from Amersham-Pharmacia (Piscataway, NJ, USA). 2.2. Cell
Culture Human neuroblastoma cell line SK-N-MC was cultured in RPMI-1640 medium
supplemented with FBS (10%, v/v), streptomycin (100 μg/ml) and penicillin (100
U/ml) and incubated in 5% CO humidified atmosphere at 37˚C. To induce oxidative
stress, menadione was freshly prepared from a stock solutions (10 mM), prior to
each experiment. Menadione and baicalein were dissolved in a minimum amount of
dimethyl Sulfoxide (DMSO) and then diluted with the culture medium to the
desired concentration. The concentration of DMSO in the culture medium kept
lower than 0.1% and the control cells were treated with the vehicle solution
containing the same amount of DMSO. 2.3. Determination of Cell Viability Cell
viability was assessed by the 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl tetrazolium
bromide (MTT) reduction assay. Viable cells with active mitochondria reduce the
yellow tetrazolium salt MTT giving dark blue water insoluble formazan crystals.
To perform the assay for evaluation of the cytoprotective effects of baicalein
and caspase inhibitor (z-VAD-fmk) on menadione-treated SK-N-MC cells, SK-N-MC
cells were suspended in medium and seeded at a density of 5 × 104 cells/well in
96 well plates for a day. Cells were pretreated with various concentrations of
baicalein (10, 20, 40, 50 μM) and pancaspase inhibitor (50 μM) and then treated
with menadione (35 μM) for additional 24 h at 37˚C. MTT was dissolved at a
concentration of 5 mg/ml in PBS and stored at 4˚C, protected from light and
tightly capped. After incubation, cells were treated with the 10 μl MTT
solution for 4 h. Then, the medium was removed and 200 μl DMSO was added to
each well. The formazan dye crystals were solubilized in 30 min, and absorbance
was measured at 570 nm using an ELISA reader (Exert 96, Asys Hitch, Ec Austria).
Results were expressed as the percentage of MTT reduction, assuming that the
absorbCopyright © 2013 SciRes. CellBio M. MOSLEHI, R. YAZDANPARAST 37 ance of
the control cells was 100%. 2.4. Measurement of Intracellular ROS Oxidation of
2’,7’-dichlorofluorescein diacetate (DCFHDA) to fluorescent DCF is taken as an
index of overall oxidative stress in biological system according to LeBel
method [24]. Cells were pre-treated with various concentrations of baicalein
(10, 20, 40 μM) and 50 μM caspase inhibitor for 3 h followed by menadione
treatment (35 μM) for 12 h at 37˚C. Then the cells were incubated with 10 μM
DCFH-DA for 1 h followed by washing twice with phosphate buffer saline and
suspension in the same buffer. Finally, the fluorescent intensity was monitored
using a varian-spectrofluorometer with excitation and emission wavelength of
485 and 530 nm, respectively. 2.5. Determination of Lipid Peroxidation
Malondialdehyde (MDA) levels were measured by the double heating method [25].
The method is based on spectrophotometric measurement of the purple color
generated by the reaction of thiobarbituric acid (TBA) with MDA. Briefly, 0.5
ml of cell lysate was mixed with 2.5 ml of trichloroacetic acid (TCA, 10%, w/v)
solution followed by boiling in a water bath at 95˚C for 15 min. After cooling
to room temperature, the samples were centrifuged at 3000 rpm for 10 min and 2
ml of each sample supernatant was transferred to a test tube containing 1 ml of
TBA solution (0.67% w/v). Each tube was then placed in a boiling water bath for
15 min. After cooling to room temperature, the absorbance was measured at 532
nm with respect to the blank solution. The protein concentration was determined
by Lowry’s method [26]. The concentration of MDA was calculated based on the
absorbance coefficient of the TBA-MDA complex (ε = 1.56 × 105 cm−1 ·M−1 ) and
it was expressed as nmol/ mg of protein. 2.6. Determination of Protein Carbonyl
Formation The assessment of protein carbonyl content is a widelyused marker for
oxidative protein modification. Protein carbonyls (PCOs) were measured using
Reznick and Packer method [27]. Briefly, 1 ml of 10 mM DNPH in 2 M HCl was
added to the cell lysates. Samples were incubated for 1 hr at room temperature
and were vortexed every 15 min. Then, 1 ml of trichloroacetic acid (TCA 10%
w/v) was added to each reaction mixture and centrifuged at 3000 rpm for 10 min.
The pellets were washed twice with 2 ml of ethanol/ethyl acetate (1:1, v/v) and
each dissolved in 1 ml of guanidine hydrochloride (6 M, pH 2.3) and incubated
for 10 min at 37˚C whilst mixing. The carbonyl content was calculated based on
the molar extinction coefficient of DNPH (ε = 2.2 × 104 cm−1 ·M−1 ). 2.7.
Fluorescence Microscopy Evaluation of Apoptotic Cells Acridine orange/ethidium
bromide double staining was applied to observe the morphological changes among
menadione-treated cell. Using this technique, cells can be distinguished as
normal cells (uniformly stained green) and apoptotic cells that are stained
orange because of cell membrane destruction and the intercalation of ethidium
bromide between the nucleotide bases of DNA. After treatment, cells were washed
twice with phosphate buffer saline and adjusted to a cell density of 1 × 104
cells/ml of phosphate solution (1:1 v/v). The nuclear morphology was evaluated
by Axoscope 2 plus fluorescence microscope from Zeiss (Germany). The cells with
condensed or fragmented nuclei were counted as apoptotic cells. All experiments
were repeated three times, and the number of stained cells was counted in 10 randomly
selected fields. 2.8. Evaluation of Intracellular Formation of Lipofuscin
Pigments Extraction of intracellular lipofuscin was achieved following lysis of
each sample according to a published procedure with slight modification [28].
The cells were seeded in triplicate into 24-well plates for 24 h prior to
pretreatments. After pretreatment with different doses of baicalein (10, 20, 40
μM) for 3 h, each cell sample was treated with 35 μM menadione for 24 h. The
attached cells in each well were trypsinized with trypsin-EDTA solution
followed by cell counting using a hemocytometer. Each plate was then
centrifuged, the cell pellet was washed with PBS, and the cell content was
lysed with lysis buffer containing 1% Triton X-100, 1 mM EDTA and 1 mM PMSF.
Each cell lysate was harvested and its fluorescence intensity was monitored on
a varian spectrofluometer, model Cary Eclipse, with an excitation wavelength of
310 nm and emission wavelength of 620 nm [29]. The fluorescence intensities of
the samples were then normalized for equal cell numbers. 2.9. Measurement of
Intracellular Iron Contents via Ferrozine-Based Colorimetric Assay The assay
was performed directly in 24-well plates. Cells were lysed by addition of 200
μl iron releasing reagent (a freshly mixed solution of equal volumes of 1.4 M
HCl and 4.5% (w/v) KMnO4 in H2O2) to each well. The plates were sealed with
foil and incubated for 2 h at 60˚C, after which 60 μl of the detection reagent
(6.5 mM ferrozine, 6.5 mM EDTA, 2.5 M ammonium acetate and 1 M ascorbic acid
dissolved in water) was added. After further incubation for 30 min at room
temperature, 280 μl of the mixture was transferred to a well of a 96-well plate
and its absorbance recorded at 550 nm and compared to the absorbance of the
FeCl3-treated standards under all Copyright © 2013 SciRes. CellBio 38 M.
MOSLEHI, R. YAZDANPARAST equal experimental conditions. The determined
intracellular iron concentration for each well was normalized against the
protein content of replicate wells [30]. 2.10. Western Blot Analysis SK-N-MC
cells were seeded at a density of 105 cells/ml in 12-well plates for 24 h. The
cells were pretreated with baicalein (40 μM) and caspase inhibitor (50 μM).
After 3 h, menadione (35 μM) was added to the cells and incubated at 37˚C for an
additional 24 h. Then, the cells were harvested and lysed using lysis buffer
containing 1% Triton X-100, 1% SDS, 10 mM Tris (pH 7.4), 100 mM NaCl, 1 mM
EGTA, 1 mM EDTA, 20 mM sodium pyrophosphate, 2 mM Na3VO4, 1 mM NaF, 0.5% sodium
deoxycholate, 10% glycerol, 1mM phenylmethylsulphonyl fluoride, 10 μg/ml
leupeptin, 1 μg/ml pepstatin and 60 μg/ml aprotinin. Protein concentration of
each sample was determined using Lowry’ method (Lowry et al., 1951). Equal
quantities of protein (40 μg) were subjected to 12.5% SDS-polyacrylamide gel
electrophoresis (PAGE) and were transferred to PVDF membranes. The blots were
blocked with 5% (w/v) non-fat dry milk in Tris-buffered saline buffer
containing 0.1% Tween-20 (TBS/T) for an overnight at 4˚C. The blocked blots
were incubated with primary antibodies for 2 hr at room temperature using
antibody dilutions as recommended by the manufacturer in Tris-buffered saline
pH 7.4 containing 0.1% Tween-20. After 1-hr incubation with anti-rabbit or
anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibodies
(Biosource), the proteins were detected by an enhanced chemiluminescence
detection system (Amersham-Pharmacia, Piscataway, NJ, USA) according to the
manufacturer’s instructions. Blots were stripped at 50˚C for 30 min in 100 mM
2-mercaptoethanol, 2% SDS, 62.2 mM Tris-HCl pH 6.7 and reprobed for further
investigations. For analysis of the western blotting data, densitometric
analysis was performed using Image.J software, and the densities were
normalized with respect to β-tubulin as the internal control. 2.11. Statistical
Analysis Data were expressed as percent of values of untreated control cells,
and each value represents the mean ± SD (n = 3). The significant differences
between the means of the treated and untreated cells were calculated by
unpaired Student’ t-test, and p-values < 0.05 were considered significant.
3. Results 3.1. Baicalein and Pan-Caspase Inhibitor (Z-VAD-Fmk) Shield SK-N-MC
Cells against Menadione-Induced Cytotoxicity Menadione is a quinone known to induce
an oxidative stress generated primarily by superoxide radicals leading to cell
death [31]. We found that menadione at 35 μM caused 55% cell death among
SK-N-MC cells (Figure 1). In our previous study, we ascertained that no
remarkable changes were seen among the cells in range of 10 - 50 μM of
baicalein after 24 h [32]. Thus, cytoprotcetive effects of different doses of
baicalein (10, 20, 40, 50 μM) on menadione (35 μM)-induced cytotoxicity in
SK-N-C cells were investigated. The detrimental effects of menadione on SK-N-MC
cells were considerably blocked by pretreatment with baicalein. The same result
was observed for Z-VAD-fmk. As shown in Figure 1, the extent of survival was
restored to 67%, 84%, 89% and 71% by pretreatment of cells with different doses
of baicalein (10, 20, 40, 50 μM) for 3 h followed by treatment with 35 μM
menadione for 24 h. Baicalein at a concentration of 40 μM, provided utmost
protection against menadione insult producing a 44% increase in cell survival.
Moreover, Z-VAD-fmk (50 μM) increased cell viability to 86% (Figure 1). 3.2.
Baicalein but Not Z-VAD-Fmk, Mitigates Menadione-Induced Increase in
Intracellular ROS Generation Increase in ROS generation was measured as one of
the indicators of menadione-induced oxidative stress in cells. As shown in
Figure 2, generation of intracellular ROS (in term of DCF fluorescent
intensity) in SK-N-MC cells increased by almost a factor of 6.2 after 12-h
treatment Figure 1. Cts of menadione, baicalein, and Z-VAD-fmk on viability of
SK-N-MC cells. SK-N-MC cells were treated with different concentrations of
menadione (20, 35, 50 μM) to find IC50 of menadione for further experiments (35
μM). Then, SK-N-MC cells were pretreated with different concentrations of
baicalein (10, 20, 40, 50 μM) and Z-VAD-fmk (50 μM) for 3h and then incubated
with menadione (35 μM) for 24 h. Cell viability was examined by MTT assay.
Values correspond to means ± SD of three independent experiments. *
significantly different from control cells (p < 0.05), # significantly different
from menadione-treated cells (p < 0.05). Copyright © 2013 SciRes. CellBio M.
MOSLEHI, R. YAZDANPARAST 39 Figure 2. Effects of baicalein and Z-VAD-fmk on
intracellular ROS level in menadione-treated SK-N-MC cells. SKN-MC cells were
pretreated with baicalein (10, 20, 40 μM) and Z-VAD-fmk (50 μM) for 3 h and
then incubated with menadione for 12 h. ROS levels were monitored using 2', 7'
dichlorofluorescein diacetate (DCFH-DA) staining. The fluorescence intensity
was monitored on a varian-spectrofluorometer with excitation and emission
wavelengths of 485 and 530 nm, respectively. Values correspond to means ± SD of
three independent experiments. * significantly different from control cells (p
< 0.05), # significantly different from menadione-treated cells (p < 0.05).
with menadione (35 μM) compared to ROS level of the untreated control cells.
Pretreatment of the cells with different doses of baicalein (10, 20, 40 μM)
attenuated ROS production in SK-N-MC cells by factors of 2.3, 3.6 and 4.3,
respectively. However, pretreatment with ZAD-mk (50 μM for 3 h) did not
significantly change the ROS level in menadione-treated SK-N-MC cells. 3.3.
Baicalein but Not Z-VAD-Fmk, Curbs Menadione-Induced Lipid Peroxidation
Menadione-induced oxidative stress causes oxidation of intracellular
biomolecules such as lipids. MDA is produced while lipid peroxidation happens.
So, MDA level measurement is used as a marker of menadione-induced oxidative
stress. As shown in Figure 3, baicalein repressed lipid peroxidation in SK-N-MC
cells. After 12 h of incubation with 35 μM menadione, MDA levels were
significantly increased relative to the untreated control cells (0.41 nmol/mg
protein in control cells versus 2.33 nmol/mg protein in menadione-treated
cells). Pretreatment of cells with different doses of baicalein (10, 20, 40 μM)
for 3 h followed by a 12 h treatment with menadione (35 μM) reduced MDA
formation to 1.64, 1.01, and 0.66 nmol/mg protein, respectively, indicating
that baicalein had quenched lipid peroxidation of the SK-NMC cells. However,
pretreatment with 50 μM Z-VADfmk did not significantly alter MDA contents in
menadione-treated SK-N-MC cells. Figure 3. Effects of baicalein and Z-VAD-fmk
on intracellular lipid peroxidation and protein carbonyl formation in
menadione-treated SK-N-MC cells. SK-N-MC cells were pretreated with baicalein
(10, 20, 40 μM) and Z-VAD-fmk (50 μM) for 3 h and then incubated with menadione
for 12 h. lipid and protein oxidations were measured by analysis of MDA and
PCO. Values correspond to means ± SD of three independent experiments. *
significantly different from control cells (p < 0.05), # significantly
different from menadionetreated cells (p < 0.05). 3.4. Baicalein but Not
Z-VAD-Fmk, Diminishes Menadione-Induced Protein Carbonyl Formation Protein carbonyl
is a marker of protein oxidation in oxidative stress condition. We evaluated
the effects of different doses of baicalein (10, 20, 40 μM) and Z-VADfmk (50
μM) on protein carbonyl formation in SK-N-MC cells. After treatment with
menadione (35 μM), the amount of protein carbonyl increased to 4.03 nmol/mg
protein compared to 0.65 nmol/mg protein of control cells. Pretreatment with
baicalein (10, 20, 40 μM) reduced protein carbonyl formation to 2.6, 1.7 and
1.1 nmol/mg protein, respectively (Figure 3). However, pretreatment with 50 μM
Z-VAD-fmk did not significantly alter PCO contents in menadione-treated SK-N-MC
cells. 3.5. Baicalein and Z-VAD-Fmk Prevent Menadione-Induced Caspase-Dependent
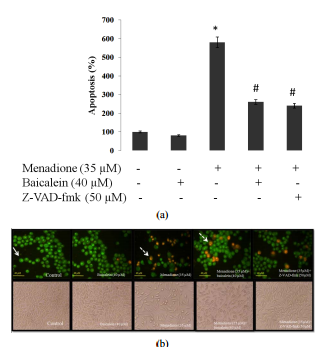
Apoptotic Cell Death To study the protective effect of baicalein on SK-N-MC
cells, acridine orange/ethidium bromide double staining technique was used to
evaluate the occurrence of apoptosis in cells. As shown in Figure 4, the
non-apoptotic control cells were stained green and the apoptotic cells had
orange particles in their nuclei due to nuclear DNA fragmentation. The
menadione treatment increased the extent of apoptosis relative to untreated
control cells and pretreatment with baicalein (40 μM, 3 h) diminished apoptosis
compared to menadione-treated cells (Figure 4). We also pretreated SK-N-MC
cells with Z-VAD-fmk (50 μM) for 3 h followed by exposure to menadione (35
Copyright © 2013 SciRes. CellBio 40 M. MOSLEHI, R. YAZDANPARAST (a) (b) Figure
4. Effect of baicalein and Z-VAD-fmk treatments on menadione-induced apoptosis
in SK-N-MC cells. (a) SKN-MC cells were treated with baicalein (40 μM) and
Z-VAD-fmk (50 μM) for 3 h followed by exposure to menadione (35 μM) for 24 h.
cell pretreatment with baicalein and Z-VAD-fmk clearly decreased the number of
apoptotic cells relative to cells treated only with menadione. Values
correspond to means ± SD of three independent experiments. * significantly
different from control cells (p < 0.05), # significantly different from
menadione-treated cells (p < 0.05); (b) morphological analysis of SK-N-MC
cells by double staining method. White arrow indicates live cells, dashed arrow
shows apoptotic cells. Scale bar: 40 μM. μM) for 24 h. As shown in Figure 4,
Z-VAD-fmk reduced the extent of apoptosis relative to menadionetreated cells,
confirming the caspase-dependent apoptosis of cells. 3.6. Effect of Baicalein
on Menadione-Induced Lipofuscin Formation Exposure of the cells to 35 μM
menadione for 24 h caused 374% increase in the intracellular level of
lipofuscin relative to menadione-untreated control cells. Pretreatment of the
cells with baicalein (10, 20, 40 μM) diminished the formation of lipofuscin
pigments by 155%, 192% and 214% after 24 h of exposure (Figure 5). 3.7.
Baicalein Decreases Iron Accumulation in Menadione-Induced SK-N-MC Cells Iron is
important for electron transport in the respiratory chain and for various
enzymatic reactions. When present in excess, however, iron can harm biological
systems since in redox-active form it catalyzes the generation of highly
reactive oxygen species [33]. Since both iron deficiency and overload impaired
cellular functions, the quantitation of iron in cells and extracellular fluids
is of considerable interest [34,35]. As shown in Figure 6, treatment of SK-N-MC
cells with menadione elevated free iron contents compare to basal iron level in
the control samples (2.17 nmol/mg proteins compare to 1.1 nmol/mg protein of
control). However, pretreatments with different doses of baicalein (10, 20, 40
μM) diminished the iron contents to 1.75, 1.54 and 1.33, respectively. 3.8.
Effects of Baicalein and Z-VAD-Fmk on Menadione-Induced Cell Death Previous
studies have shown that menadione-induced Figure 5. Inhibitory effect of
baicalein on the menadionetreated accumulation of intracellular lipofuscin
pigments. SK-N-MC cells were exposed to baicalein (10, 20, 40 μM) for 3 h
followed by exposure to menadione (35 μM) for 24 h. Then, the extent of
lipofuscin in cell lysates were evaluated using a varian spectrofluorometer,
model Cary Eclipse, set at an excitation wavelength of 310 nm and an emission
wavelength of 620 nm. * significantly different from control cells (p <
0.05), # significantly different from menadionetreated cells (p < 0.05).
Figure 6. Effect of baicalein on intracellular iron contents in
menadione-treated SK-N-MC cells. SK-N-MC cells were exposed to baicalein (10,
20, 40 μM) for 3 h followed by exposure to menadione (35 μM) for 24 h. Iron
contents were evaluated by colorimetric ferrozine-based assay. * significantly
different from control cells (p < 0.05), # significantly different from
menadione-treated cells (p < 0.05). Copyright © 2013 SciRes. CellBio M.
MOSLEHI, R. YAZDANPARAST 41 apoptosis is associated with changes in
apoptosis-related Bcl-2 family of regulatory proteins. Bax is a pro-apoptotic
member of the Bcl-2 family which forms mitochondrial permeability pores for
release of cytochrome c to the cytosol via binding to the anti-apoptotic Bcl-2
member. This event in turn will lead to cleavage of procaspase-9 and further
activation of procaspase-3 and cell death through apoptosis [36]. Pretreatment
of cells with baicalein prior to menadione treatment, reduced Bax/ Bcl2 ratio
and pretreatment of cells with baicalein and Z-VAD-fmk decreased cleaved
caspase-9 in SK-N-MC cells which showed that baicalein inhibited
caspase-dependent apoptosis in this cell line (Figure 7). 4. Discussion One of
the well-accepted theories for explicating the aging process is the free
radical theory proposed by Denham Harman [5]. This theory illustrates that
there is a causal relationship between oxidative stress and pathogenesis of
age-related disorders [6]. Lipofuscin, a histological index of aging, is a
highly oxidized cross-link aggregate consisting of oxidized proteins (30% -
58%) and lipids (19% - 51%) clusters accrues mostly in postmitotic cells such
as neurons, cardiac myocytes, skeletal muscle fibers and retinal pigments [7].
Since oxidative reactions are compulsory components of normal life processes,
the incidence of reactive oxygen species with ensuing lipofuscin formation is an
inexorable side effect of life [10]. Many studies have signified that many
ROSinduced diseases such as neurodegenerative disorders are associated with
high levels of lipofuscin within neuronal cells [37,38]. It has been widely
reported that loosely bound iron in the cellular iron pool can react with
endogenous hydrogen peroxide to produce the short-lived and highly reactive
hydroxyl radicals through the Fenton reaction. These hydroxyl radicals, in
turn, can oxidize nucleic acids, proteins or lipids leading to lipofuscin
formation [23]. Oxidized proteins within lipofuscin are linked by
intermolecular cross-links. Many of these cross-links are caused by non
proteineous compounds including oxidized lipids such as Malondialdehyde (MDA)
and 4-hydroxy-2-nonenal by means of reactions with lysine amino groups,
cysteine sulfhydryl groups and histidine imidazole groups of proteins [39].
Thus, preventing biomolecules peroxidations and maintaining iron homeostasis
play major roles in blocking lipofuscin formation. Menadione (2-methyl-1,4
naphthoquinone) in the cells converts to menadione semiquinone radical via
NADPH cytochrome c reductase activity. Then, semiquinone radical is recycled
back to menadione through rapid reaction with molecular oxygen. This can result
in the formation of superoxide radical which causes oxidative stress [40].
Although superoxide is chemically incapable of (a) (b) Figure 7. Analysis of
Bcl-2, Bax and procaspase-9 activation in SK-N-MC cells treated with menadione,
baicalein and Z-VAD-fmk. SK-N-MC cells were pretreated with baicalein (40 μM)
and Z-VAD-fmk (50 μM) for 3 h and then incubated with menadione (35 μM) for 24
h. (a) bcl-2, Bax and the (b) procaspase-9 expression were estimated by
immunoblots using relevant specific antibodies, and intensity of each band was
estimated by densitometric analysis. Equal sample loadings were confirmed by
tubulin band. Values correspond to means ± SD of three independent experiments.
* significantly different from control cells (p < 0.05), # significantly different
from menadione-treated cells (p < 0.05). affecting biomolecules directly, it
is assumed to do so indirectly by participating in the production of hydroxyl
radicals through Fenton reaction. Superoxide radicals can provide free iron to
catalyze peroxidation from two sources: release iron from ferritin and oxidizes
the [4Fe - Copyright © 2013 SciRes. CellBio 42 M. MOSLEHI, R. YAZDANPARAST 4S]
clusters of enzymes such as dehydratases, precipitating the release of one or
more iron atoms [41]. Thus, menadione as a Fenton catalyst, assisted the
production of free iron for production of hydroxyl radicals to ignite cross
link of oxidized proteins and lipids in order to form lipofuscin. There is an
accumulating evidence denoting that lipofuscin can induce neurotoxicity via its
capacity for binding metals such as iron, copper, zinc and calcium which
stimulates generation of excessive ROS and decrease proteasomal and lysosomal
degradation by inhibition of the proteasomal turnover [7]. Numerous studies
have shown that intracellular iron accumulation contributes to the development
of several common neurodegenerative diseases such as Alzheimer’s disease (AD)
and Parkinson’s disease (PD) [33-35]. In order to restrain the destructive
effects of ROS including superoxide radicals in neuronal cells, dietary
flavonoids are shown to have potential anti-aging and brain-protective
activities. Baicalein (5, 6, 7-trihydroxy- 2-phenyl-4H-1-benzopyran-4-one), a
naturally occurring flavonoid, is the major bioactive compounds found in
traditional Chinese medicinal herb, Baikal Skullcap (Scutellaria baicalensis
GEORGI) [22]. Baicalein produces promising results as a strong antioxidant. Its
ability to cross blood brain barrier (BBB), hydrophobicity, presence of
hydroxyl groups at C-5 and C-7, a double bond between C-2 and C-3, high trolox
equivalent antioxidant capacity (TEAC) and DPPH free radical scavenging
activity make baicalein a good ROS scavenger in neurons [20,21]. Presence of
hydroxyl groups in baicalein structure results in scavenging of charged species
such as superoxide radicals and hydroxyl radicals more efficiently compared to
non-charged oxidant species [20]. On the other hand, baicalein can inhibit the
production of endogenous hydroxyl radicals produced through the Fenton reaction
by forming stable and inert complexes with iron [23]. Iron-binding motifs in
some phenolic compounds can clarify the potential ability of them to modulate
iron homeostasis in the body. Baicalein contains these motifs and thus expected
to chelate iron. Some recent studies have shown that two hydroxyl groups at the
6 and 7 positions on the A ring seems to be the powerful metal binding site
[20,23]. In support of what we have explained before, our studies showed that
baicalein reduced the harmful effects of menadione by scavenging superoxide
radicals which led to increased cell viability and decreased intracellular MDA
and PCO. In addition, our results confirmed that baicalein has anti-Fenton
properties since it decreased the free iron contents of SK-N-MC cells exposed
to menadion treatment. We also observed that baicalein strongly inhibited
lipofuscin formation in menadione-treated SKN-MC cells and displays anti-aging
features. Morphological analysis and western blot results implied that
baicalein prevented apoptotic cell death through inhibition of Bax and
procaspase-9 activations and induction of bcl2 expression which averted
activation of further caspases and transcription factors, release of cytochrome
c and resultant cell death. The results were confirmed by applying pan-caspase
inhibitor (Z-VAD-fmk). Moreover, our experiments have shown that Z-VAD-fmk
prevented cell death in SK-N-MC cells through inhibition of caspases and did
not have any significant antioxidant characteristics. Overall, flavonoid
baicalein can be considered as a strong and auspicious antioxidant which could
protect neuronal cells and hence, baicalein is a reliable option for
antioxidant therapy in treatment of age-related and neurodegenerative
disorders, pending further in vivo and clinical investigations. 5.
Acknowledgements The author appreciates the financial support of this
investigation by the Research Council of University of Theran.