Analyzing Nootropic Effect of Phyllanthus
reticulatus Poir. on Cognitive Functions,
Brain Antioxidant Enzymes and
Acetylcholinesterase Activity against
Aluminium-Induced Alzheimer’s Model in
Rats: Applicable for Controlling the Risk
Factors of Alzheimer’s Disease
Md. Sahab Uddin 1* , Abdullah Al Mamun 1 , Mohammed Ashraful Iqbal 2 , Ariful Islam 3 ,
Md. Farhad Hossain 1 , Sayema Khanum 1 , Mamunur Rashid 1,4
1 Department of Pharmacy, Southeast University, Dhaka, Bangladesh
2 Department of Chemistry, Fareast International University, Dhaka, Bangladesh
3 Department of Pharmacy, State University of Bangladesh, Dhaka, Bangladesh
4 Department of Pharmacy, University of Rajshahi, Rajshahi, Bangladesh
Received 6 March 2016; accepted 7 July 2016; published 21 July 2016
Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/Abstract
Oxidative stress is intensely linked with neurodegenerative disorders, especially Alzheimer’s dis-
ease (AD). Searching for medicinal plant with the nootropic activity for controling the develop-
ment and progression of AD has received extensive consideration. The plant Phyllanthus reticula-
tus (PR) Poir. is known in Bengali as Panjuli belongs to family Euphorbiaceae. Previous studies
have shown the antioxidant, analgesic, anti-inflammatory, etc. activities of this plant. Therefore,
the objective of this study was to examine the nootropic effect of ethanolic extracts of Phyllanthus
reticulatus (EEPR) on cognitive functions, brain antioxidatant enzymes and acetylcholinesterase
activity in aluminium-induced rats of cognitive impairment and oxidative stress. The effects of
EEPR fruit (i.e., 100 and 200 mg/kg b.w.) were examined for 30 days and its nootropic effect was
determined in aluminium treated Swiss albino male rats by behavioral studies such as Passive
Avoidance (PA) test, Rewarded Alternation (RA) test and biochemical studies such as superoxide
* Corresponding author.
Md. S. Uddin et al.
88
dismutase (SOD), catalase (CAT), contents of thiobarbituric acid reactive substances (TBARS) and
acetylcholinesterase (AChE) activity in rats brain tissue homogenates. In PA test, administration of
EEPR fruit (i.e., 100 and 200 mg/kg, b.w.) significantly (P < 0.05, P < 0.01) increased step-through
latency (STL) in rats on 30 th day with respect to disease control group. The percentage of memory
retention (MR) for this test was pointedly (P < 0.05) increased in rats treated with EEPR fruit (i.e.,
200 mg/kg b.w.) as compared with disease control group. For RA test, EEPR fruit (i.e., 200 mg/kg
b.w.) markedly (P < 0.01) increased the correct responses (CR) in rats on 30 th day related to dis-
ease control group. In case of this test the percentage of MR was significantly (P < 0.05, P < 0.01)
increased in rats treated with EEPR fruit (i.e., 100 and 200 mg/kg b.w.) with respect to disease
control group. Administration of EEPR fruit (i.e., 100 and 200 mg/kg b.w.) considerably (P < 0.05, P
< 0.01) increased the level of SOD, CAT and expressively (P < 0.05) decreased TBARS level com-
pared to disease control group. Treatment with EEPR fruit (i.e., 100 and 200 mg/kg b.w.) markedly
(P < 0.05, P < 0.01) decreased the level of AChE activity to that of disease control group. The
present study shows that EEPR fruit has excellent nootropıc effect on cognitive performance and
brain antioxidant markers in aluminium-induced rats of cognitive impairment and oxidative stress
which could be developed in the management of neurodegenerative diseases especially AD.
Keywords
Nootropic, Phyllanthus reticulatus, Cognitive Functions, Brain Antioxidant Enzymes,
Acetylcholinesterase Activity, Alzheimer’s Disease
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative brain disease that causes problems with memory,
thinking, behavior and finally lead to death [1]. It is the most common form of dementia (60%) which is not a
normal part of aging [2]. AD is characterized by the presence of excessive amounts of neuritic plaques contain-
ing amyloid-β (Aβ) and abnormal tau protein filaments in the form of neurofibrillary tangles (NFTs) in the cere-
bral cortex and subcortical gray matter [3]. The brain of Alzheimer patient’s has increased levels of acetylcholi-
nesterase (AChE), which is accountable for the breakdown of acetylcholine (ACh) [4]. ACh is a neurotransmit-
ter which plays a pivotal role for the appropriate functioning of the central cholinergic system (CCS) [5]. A di-
minution of ACh in the brain of patients with AD appears to be a foremost reason in producing dementia [6].
Worldwide at present about 35 million people are affected by AD and it is the 6 th foremost cause of death in the
United States [7]. Patients with Alzheimer’s may live an average of 8 years after their symptoms become consi-
derable to others. However, survival range can be diverse from 4 to 20 years which is depending on age and
other health conditions. The current Alzheimer’s treatments cannot discontinue the progression of this neurode-
generative disease, but these can temporarily slow down the worsening of dementia symptoms and ameliorate
the quality of life in Alzheimer’s patients [8]. Today the scientists have been working relentlessly and providing
their maximum effort to find better ways to treat the disease, delay its onset and prevent it from developing [9].
Oxidative stress occurs when free radicals and their by-products are produced in excessive amount compare to
antioxidant defense mechanisms [10]-[12]. The pathogenesis of AD leading to neuronal dysfunction and cell
death, mainly due to imbalance between free radical production and antioxidant defenses [13] [14]. Research has
recently displayed that brain tissue in patients with AD is exposed to protein oxidation, DNA oxidation, lipid
oxidation, glycoxidation etc. during the period of the disease [15] [16]. The oxidation of proteins by free radicals
plays a significant role in AD [17]. Several studies exhibit an augment in protein carbonyls in multiple brain
areas in subjects with AD [18]. Enzyme mainly glutamine synthetase and creatine kinase are sensitive to oxida-
tive modification and noticeably reduced in the brains of Alzheimer’s patients [19]. Higher levels of lipid pe-
roxidation take place in the brain in AD and are most well-known where degenerative changes are most noticea-
ble [20]. Polyunsaturated fatty acids (PUFAs) of the brain membrane phospholipids are particularly susceptible
to free radical attack because their double bonds permit easy withdrawal of hydrogen ions [21]. Oxidation of
PUFAs, principally arachidonic and docosahexaenoic convey lipid peroxidation in AD [22] by generating alde-
Md. S. Uddin et al.
89
hydes most importantly 4-hydroxynonenal (HNE), a highly reactive cytotoxic agent able to inhibit glycolysis,
nucleic acid and protein synthesis and degrading proteins [23]. Oxidation of DNA can generate strand breaks,
sister chromatid exchange, DNA-protein crosslinking and base alterations [24]. Numerous studies exhibit an in-
crease in oxidative DNA damage in the brain’s of subjects with AD [25]. The greatest marked DNA adduct de-
fined is 8-hydroxy-2-deoxyguanosine (8-OHdG) [26]. On the other hand advanced glycation end products are
produced due to posttranslational modifications of proteins and may play a role in AD that is connected to oxid-
ative modifications of Aβ peptides and tau [27]. In addition to this, the brain is largely composed of easily oxi-
dized lipids, has a high oxygen consumption rate, high metabolic rate of transitional metals and lacks strong an-
tioxidant defenses, that’s why it is quite vulnerable to oxidative injury [28].
Aluminium is a generally manifested neurotoxin and possesses diverse mode of action on the central nervous
system (CNS) [29]. It is able to rise the permeability and crossing of the blood-brain barrier (BBB) [30] [31],
which plays a significant role to increase the concentration of aluminium in the hippocampus [32], cortex, sin-
gulated bundles and corpus callosum [33]. Various neuropathological, biochemical and epidemiological studies
have recommended a potential connection between the pathogenesis of AD and neurotoxicity of aluminium [34].
Aluminium develops accumulation of insoluble Aβ, aggregation of hyper phosphorylated tau protein which
contains NFTs [35] and causes harmful alteration to cholinergic neurotransmission [36]. Furthermore, alumi-
nium promotes oxidation triggered by various transition metals such as chromium (Cr) and copper (Cu) [37]. In
this study to created cognitive dysfunction and oxidative stress aluminium maltolate was used. The advancement
of drugs for the treatment of AD that breaks the vicious cycles of oxidative stress and neurodegeneration re-
commends new prospects.
Antioxidants are agents that are the vital part of most favorable health and able to prevent or delay some kinds
of cell damage [38]. Several scientific researchers recommended that antioxidants play a central role in the
management of AD. Naturally occurring antioxidants are extremely useful for AD in order to reduce risk con-
nected with synthetic antioxidants [39]. The greatest origin of natural antioxidant is medicinal plants. The neu-
roprotective effects of natural antioxidants and nootrpics, such as Ginkgo biloba, [40] Bacopa monnieri [41] and
Huperzia serrata has [42] attained considerable attention in the management of AD.
The plant Phyllanthus reticulatus (PR) Poir. is known in Bengali as Panjuli belongs to Euphorbiaceae family
[43]. This plant is extensively distributed throughout the tropical areas of India, China, Malay Islands and fallow
lands of Bangladesh [44]. The fruit of this plant is roundish berry with a diameter of about 4 to 6 mm, green in
color at first and becomes purplish black [45]. In the traditional system of medicine different parts of this plant
are used for curing various diseases. Leaves are used as antidiarrheal, diuretic, cooling medicine, roots are used
for treating malaria, asthma and bark is used as astringent and diuretic. The fruit of this plant shows astringent
properties to the bowels and used in inflammation [43]. The important therapeutic uses of this plant are analges-
ic, anti-inflammatory, hypocholesterolemic, cytotoxic, immunostimulant, antidiabetic, antiplasmodial, antimi-
crobial, hepatoprotective activities etc. [46]. The chemical studies of this plant ensured the presence of following
phytoconstituents including tannic acid, octacosanol, sitosterol, scopoletin, lupeol acetate, teraxerone, betulin,
teraxerol acetate, friedeline, stigamasterol and lupeol [47] [48].
Previous preliminary studies suggested in vitro antioxidant activity of this plant [49]. Consequently, the inten-
tion of this study was to investigate the neuroprotective effect of ethanolic extract of PR (EEPR) fruits on alu-
minium-induced rats of cognitive impairment and oxidative stress by behavioral tests such as Passive Avoidance
(PA) test, Rewarded Alternation (RA) test and the activity of brain antioxidant enzymes by biochemical tests
such as superoxide dismutase (SOD), catalase (CAT), estimation of contents of thiobarbituric acid reactive sub-
stances (TBARS) and acetylcholinesterase (AChE) activity in rat brain tissue homogenates.
2. Materials and Methods
2.1. Chemicals and Drugs
Aluminium maltolate, phenazinemethosulphate, sodium pyrophosphate, nicotinamide adenine dinucleotide phos-
phate (NADPH), acetyl thiocholine iodide (ATCI), 5,5-dithiobis-2-nitrobenzoate ion (DTNB), trisamino me-
thane hydrochloride (Tris-HCl), bovine serum albumin (BSA), trichloroacetic acid (TCA) and thiobarbituric ac-
id (TBA) all were purchased from Sigma-Aldrich, USA. All other chemicals were of analytical grade, unless
otherwise specified and purchased from indigenous sources. Donepezil hydrochloride powder was obtained as
gift from Incepta Pharmaceuticals Ltd., Bangladesh.
Md. S. Uddin et al.
90
2.2. Collection and Identification of Plant Materials
The fruits of PR were collected from the Kasba, Brahmanbaria, Bangladesh, in June, 2015. The identification of
the plant was done by the expert of Bangladesh National Herbarium, Mirpur, Dhaka, Bangladesh. Accession
number: DACB-41509 for PR.
2.3. Drying and Grinding of Plant Materials
The fruits of PR weighing of about 6 kg were washed appropriately to remove dirty materials and shade dried
for 30 min. Then the fruits were permitted to shade dried for several days with irregular sun drying. Afterward,
these were dried in an oven for 24 hrs at considerably lower temperatures for the purpose of better grinding. By
using a suitable grinder the dried fruits were ground into coarse powder.
2.4. Extraction of Plant Materials
Powdered plant material (fruits) weighing of about 450 g was taken in an amber colored glass bottle and soaked
in 2 liter of 98% ethanol. The bottle with its contents was sealed and allowed to occasional shaking and stirring
at room temperature (25˚C) over a period of 7 days. After 7 days ethanol containing the extract was filtered
through cotton and then through Whatman No. 1 filter paper. After finishing the filtration the obtained liquid fil-
trates of the extract was permitted to concentrate and evaporate by drying at 45˚C temperature using a rotary
evaporator under reduced pressure to become the crude extract (11.47 g). Lastly dried crude ethanolic fruit ex-
tracts were kept in refrigerator at 4˚C until further experiments.
2.5. Animals
In this experiment 50 healthy adult male Swiss albino rats of about 200 - 230 g was acquired from ICDDR,B,
Dhaka, Bangladesh. The rats were kept in 6 per animal polypropylene cage and located under standard envi-
ronmental conditions (25˚C ± 2˚C temperature, 60% ± 5% relative humidity) with lighting (light/dark 12:12 hrs)
and sufficient supply of food and water. The animals use and care was maintained as per guidelines for labora-
tory animals of the National Institutes of Health (NIH) [50]. The protocol of the experiment was approved by the
animal ethics committee of the Department of Pharmacy, Southeast University, Dhaka, Bangladesh.
2.6. Administration of Drugs and Test Compounds
A solution of donepezil hydrochloride was prepared by using 0.9% saline solution (pH 7.4) and allowed to ad-
minister orally to experimental rats at 1 mg/kg body weight (b.w.). Aluminium maltolate was dissolved in 0.9%
saline solution (pH 7.4) and administered orally at the dose of 10 mg/kg b.w. for one month to rats. The suspen-
sion of EEPR was made by using 0.9% saline solution (pH 7.4) and orally administered at 100 and 200 mg/kg
for one month to rats. The duration of this study and doses of the donepezil, aluminium maltolate and EEPR we
reselected according to the literature review [46] [51] [52]. Suspension of the extract, donepezil and aluminium
maltolate were freshly prepared everyday and administered once daily at 10:00 am.
2.7. Experimental Design
In this experiment rats were divided into six groups and each group contains 6 rats as follows:
Group 1: Standard food and water were administered for one month to rats (Con).
Group 2: Aluminium maltolate at a dose of 10 mg/kg b.w. was administered orally for one month to rats (Alu).
Group 3: Donepezil hydrochloride at a dose of 1 mg/kg b.w. was administered orally for one month to rats
(Don).
Group 4: Aluminium maltolate at a dose of 10 mg/kg b.w. + Plant extract at a dose of 100 mg/kg b.w. were
administered orally for one month to rats (Alu + EEPR 100).
Group 5: Aluminium maltolate at a dose of 10 mg/kg b.w. + Plant extract at a dose of 200 mg/kg b.w. were
administered orally for one month to rats (Alu + EEPR 200).
Group 6: Aluminium maltolate at a dose of 10 mg/kg b.w. + Donepezil hydrochloride at a dose of 1 mg/kg
b.w. were administered orally for one month to rats (Alu + Don).
Md. S. Uddin et al.
91
2.8. Acute Toxicity Study
Acute toxicity study was accomplished as per guidelines of the Organisation for Economic Cooperation and
Development (OECD) [53]. For this test rats were separated into 6 groups, with 6 rats per groups. The fruit ex-
tract was administered orally to rats only once at a dose of 100, 200, 500, 1000, 1500 and 2000 mg/kg b.w. by
using intragastric tube. Before administration of the extracts rats were fasted for 3 to 4 hrs and after administra-
tion food was withdraw for 1 to 2 hrs. But only water was supplied continuously. The rats were closely observed
for next 24 hrs for any behavioral, neurological toxicity and 14 days for possible mortality.
2.9. Behavioral Study
Before treatment the rats were trained for 1 week to familiarize with the apparatus and in this period they did not
receive any plant extract or drug. Experiments were performed in the light period between 10:00 am and 03:00
pm in a soundproof room.
2.9.1. Passive Avoidance (PA) Test
The PA test was performed for the determination of the sensitive memory of rats depends on contextual fear
conditioning learning and instrumental learning [54]. The apparatus of PA test was made up of light and dark
compartment, each measuring 270 (depth) × 370 (width) × 360 (height) mm. In the middle part of the two com-
partments of this apparatus were linked by a sliding door having 90 mm of diameter. The floor of this apparatus
was made up of a metal grid with spaced 0.9 cm separately and joined to a shock generator able to generate
shock in the range of 0.5 mA. Fluorescent lamp was used to provide lighting in the light compartment [55]. Each
test comprises of two distinct trials including acquisition trial and retention trial. For the acquisition trial, each
rat was positioned in the light compartment fronting the wall opposed to the sliding door. The sliding door was
opened and when the rat entered into the dark compartment an electrical foot shock was provided for 3 sec after
adaptation period of 15 sec [56]. The latency times, once the rat had entered the dark compartment was recorded
as initial transfer latency (ITL) with the help of stopwatch. Then the rat was returned to its home cage. A reten-
tion trial was carried out after 24 hrs of the acquisition trial, in which no shock was given when the rat entered
the dark compartment and latency times to re-enter the dark chamber was considered as step-through latency
(STL) up to 300 sec [45]. In this study the number of ITL and STL were determined on 29 th and 30 th day respec-
tively. Based on ITL and STL the percentage of memory retention (MR) was calculated by using the formula
given below:
% MR = (STL − ITL)/ITL × 100
An increase in percentage of MR indicated improved retention of memory [57]. The apparatus was cleaned
after each test with 70% ethanol to remove any olfactory clue [58].
2.9.2. Rewarded Alternation (RA) Test
The RA test was carried out for the determination of the spatial working memory of rats [59]. The apparatus of
RA test was made up of three identical arms, each measuring 500 (length) × 100 (width) × 100 (height of the
side walls) mm. The arms were linked by a central square in the middle of the maze so as to form a T shape.
These three arms were denoted as start arm, force arm and novel arm. During the test, each rat was subjected to
6 trials and each test comprises of two separate trials including forced run trial and a choice run trial. For the
forced run trial, novel arm was blocked and each rat was positioned in the start arm facing toward the central
square and forced to the force arm owing to consume the pellet located previously. After that the rat was re-
turned to its home cage. A choice run trial was carried out after 60 sec of the forced run trial, in which novel arm
was opened (i.e., both the arms were free for the rat to choose). In this choice run trial, force arm was kept emp-
ty and pellets were positioned in the novel arm. During the choice run trial, if the rat entered into the novel arm,
then the response was measured as correct response (CR). If the rats entered into the force arm, then it was con-
sidered as a wrong response (WR) [60] [61]. In this study the number of CR and WR were determined on 30 th
day. Based on CR and WR the percentage of MR (i.e., learned task) was calculated by using the formula given
below:
% MR = TCRs × 100/TTs
where, TCRs = Total number of correct responses, TTs = Total number of trials. An increase in percentage of
MR was considered as an index of improved cognition [60] [61]. The apparatus was cleaned after each test with
Md. S. Uddin et al.
92
70% ethanol to remove any olfactory clue [58].
2.10. Biochemical Study
After 30 th days of treatment period on the next day, with the help of anesthesia the rats from all the experimental
groups were sacrificed. The entire brain was detached from the skull and then cerebellum was separated after-
ward remaining brain portion (i.e., brain portion without cerebellum) was washed with ice-cold 0.9% NaCl and
finally each hemisphere was separated. Then by using one of the two hemispheres, a 10% brain homogenate was
made by using ice-cold 30 mM phosphate buffer (pH 7.6) in a homogenizer. The homogenates were permitted to
centrifuge at 20000 RPM for 30 min at 4˚C to get homogenates which were free from any types of cell debris
and the resultant supernatant was used for the estimation of SOD and CAT. Residual hemispheres were homo-
genized (10% w/v) by using a glass homogenizer in ice-cold 30 mM phosphate buffer (pH 7.6) and allowed to
centrifuge at 20000 RPM for 2 hrs at 4˚C to get the salt soluble (SS) portion. The pellets were re-extracted with
an equivalent volume of ice-cold phosphate buffer comprising 1% Triton X-100 and permitted to centrifuge at
20000 RPM for 2 hrs at 4˚C to get the detergent soluble (DS) portion [62]. For determining the AChE activity
supernatant obtaining from both extraction processes were stored at −20˚C. The protein concentration was
measured with the help of bovine serum albumin (BSA) [63].
2.10.1. Super Oxide Dismutase (SOD) Assay
The SOD activity was determined according to the method of Kakkar et al., [64]. The total volume of the reac-
tion mixture for this test was 1.6 ml, contained 0.1 ml of 186 μM phenazinemethosulphate, 1.2 ml of 0.052 mM
sodium pyrophosphate buffer (pH 7.0) and 0.3 ml of supernatant after centrifugation (1500 × g, 10 min followed
by 10000 × g, 15 min) of 10% brain tissue homogenate. In order to start enzyme reaction 0.2 ml of 780 μM
NADH was added to the reaction mixture. The enzyme reaction was stopped by adding 1 ml of glacial acetic
acid after 1 min incubation period. The changes in absorbance of the reaction mixture were determined at 560
nm by the help of spectrophotometer and represented as U/mg protein.
2.10.2. Catalase (CAT) Assay
The CAT activity was determined according to the method of Chance and Maehly with slight modification [65].
For this test the total volume of the reaction mixture was 3.0 ml, contained 2.5 ml of 50 mM phosphate buffer
(pH 5.0), 0.4 ml of 5.9 mM hydrogen peroxide and 0.1 ml of 10% brain tissue homogenate. Then the reaction
mixture was allowed to incubate for 1 min and subsequently by the help of spectrophotometer the changes in
absorbance of the reaction mixture was measured at 240 nm. Here one unit of CAT activity was denoted as an
absorbance change of 0.01 as U/min.
2.10.3. Lipid Peroxidation (TBARS) Assay
The TBARS activity was determined according to the method of Iqbal et al., [66]. The total volume of the reac-
tion mixture was 1.0 ml, made up of 0.58 ml of 0.1 M phosphate buffer (pH 7.4), 0.2 ml of 100 mM ascorbic
acid, 0.02 ml of 100 mM ferric chloride and 0.2 ml of 10% brain tissue homogenate. The reaction mixture was
permitted to incubate at 37˚C in a shaking water bath for 1 hrs. Then 1.0 ml of 10% TCA was added to discon-
tinue the reaction. Subsequently the addition of 1.0 ml 0.67% TBA, all the test tubes was boiled in a water-bath
for 20 min. Then the test tubes were transferred to crushed ice-bath before centrifuging (2500 × g for 10 min).
The quantity of TBARS formed in each of the samples was measured by determining the optical density of the
supernatant at 535 nm by the help of spectrophotometer against a reagent blank and represented as nM
TBARS/min/mg protein at 37˚C using a molar extinction coefficient of 1.56 × 10 5 M −1 ∙cm −1 .
2.10.4. Acetylcholinesterase (AChE) Assay
The AChE activity was determined according to the method of Ellman et al., [67]. For this test, 25 μl of 15 mM
ATCI, 75 μl of 3 mM DTNB and 75 μl of 50 mM Tris-HCl (pH 8.0), containing 0.1% BSA were added in the
96 well plates and incubated for 5 min at 25˚C. Then the absorbance was measured at 405 nm by using spectro-
photometer. Any increase in the absorbance on account of the regular hydrolysis of the substrate was regulated
by deducting the rate of the reaction prior to adding the enzyme. Afterward 25 μl of brain tissue homogenates
(i.e., SS and DS portion) was added and the absorbance was measured again after incubation period of 5 min at
25˚C. The AChE activity was represented as M/min/g protein.
Md. S. Uddin et al.
93
2.11. Statistical Analysis
The results were expressed as mean ± SEM and analyzed with one-way analysis of variance (ANOVA). Tukey’s
post hoc test were performed for behavioral studies and in case of biochemical studies the least significant dif-
ference (LSD) was determined using post hoc testing for inter group comparisons at a probability level of 0.05%
and 0.01%. SPSS 14.0 (Chicago, IL, USA) and Microsoft Excel 2010 (Roselle, IL, USA) was used for the sta-
tistical and graphical evaluations. The results were considered as statistically significant at P < 0.05 compared to
disease control group.
3. Results
3.1. Determination of Acute Toxicity
EEPR up to a dose level of 2000 mg/kg b.w. had no harmful effect on the behavioral, motor and neuronal reac-
tions of the experimental rats up to 14 days of observation. Diverse doses of EEPR exhibited that there were no
signs of alters in the skin, eyes, fur and body weight therefore the extracts were considered safe.
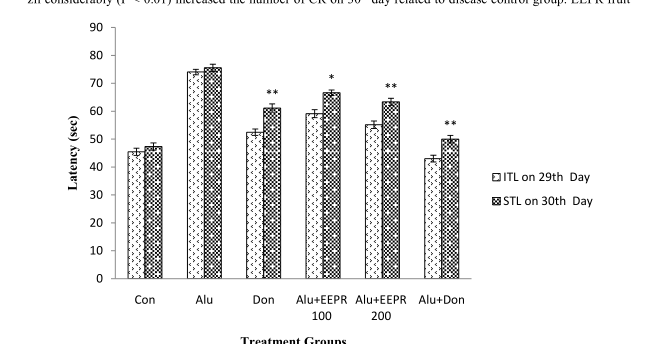
3.2. Nootropic Effect of EEPR on Learning and Memory of Rats Using PA Test
In PA test the ITL was measured on 29 th day and STL was measured on 30 th day (after 24 hrs of ITL) specified
in Figure 1. The rats treated with EEPR fruit (i.e., 100 and 200 mg/kg, b.w.) significantly (P < 0.05, P < 0.01)
increased the STL in rats on 30 th day as compared to disease control group. Treatment with donepezil sugges-
tively (P < 0.01) increased the STL of rats on 30 th day with respect to disease control group. Percentage of MR
of rats is given in Figure 2 in which an increase in MR indicated improved learning and memory of rats. The
percentage of MR was pointedly (P < 0.05) increased in rats treated with EEPR fruit (i.e., 200 mg/kg b.w.) as
compared with disease control group.
3.3. Nootropic Effect of EEPR on Learning and Memory of Rats Using RA Test
In RA test, the number of WR and CR were measured on 30 th day given in Figure 3. Administration of donepe-
zil considerably (P < 0.01) increased the number of CR on 30 th day related to disease control group. EEPR fruit
Figure 1. Nootropic Effect of EEPR on ITL and STL of rats on 29 th and 30 th day using PA test. Values were
expressed as mean ± SEM (n = 6/group). * P < 0.05, ** P < 0.01 significant difference from the disease control
group.
**
*
**
**
0
10
20
30
40
50
60
70
80
90
Con Alu Don Alu+EEPR
100
Alu+EEPR
200
Alu+Don
Latency (sec)
Treatment Groups
ITL on 29th Day
STL on 30th Day
Md. S. Uddin et al.
94
Figure 2. Nootropic Effect of EEPR on percentage of MR of rats using PA test. Values were expressed as mean ±
SEM (n = 6/group). * P < 0.05, ** P < 0.01 significant difference from the disease control group.
Figure 3. Nootropic Effect of EEPR on WR and CR of rats on 30 th day using RA test. Values were expressed as mean
± SEM (n = 6/group). * P < 0.05, ** P < 0.01 significant difference from the disease control group.
(i.e., 200 mg/kg b.w.) considerably (P < 0.01) increased the number of CR on 30 th day with respect to disease
control group. Figure 4 presented the percentage of MR of rats in which EEPR fruit (i.e., 100 and 200 mg/kg
b.w.) treated rats showed considerably (P < 0.05, P < 0.01) increased in the percentage of MR as compared to
disease control group.
**
*
*
0
2
4
6
8
10
12
14
16
18
Con Alu Don Alu+EEPR
100
Alu+EEPR
200
Alu+Don
Memory Retention (%)
Treatment Groups
**
**
**
0
1
2
3
4
5
6
Con Alu Don Alu+EEPR
100
Alu+EEPR
200
Alu+Don
Response (sec)
Treatment Groups
WR on 30th Day
CR on 30th Day
Md. S. Uddin et al.
95
Figure 4. Nootropic Effect of EEPR on percentage of MR of rats using RA test. Values were ex-
pressed as mean ± SEM (n = 6/group). * P < 0.05, ** P < 0.01 significant difference from the disease
control group.
3.4. Nootropic Effect of EEPR on Brain Oxidative Status
Table 1 represents the alteration of antioxidant enzyme activities in rat’s brain tissue homogenates. The rats
treated with EEPR fruit (i.e., 100 and 200 mg/kg b.w.) significantly (P < 0.05, P < 0.01) increased the level of
SOD and CAT but markedly (P < 0.05) reduced the concentration of TBARS to that of the disease control group.
Treatment with donepezil considerably (P < 0.05, P < 0.01) increased the level of SOD and CAT as well as
meaningfully (P < 0.05, P < 0.01) decreased the level of TBARS with respect to the disease control.
3.5. Nootropic Effect of EEPR on Brain AChE Activity
The activity of AChE in SS and DS portion of rat brain tissue homogenate is given in Table 2. Donepezil treated
group showed significantly (P < 0.05, P < 0.01) decreased in the brain AChE activity in both SS and DS por-
tions of brain tissue homogenate with respect to disease control group. Administration of EEPR fruit (i.e., 100
and 200 mg/kg b.w.) suggestively (P < 0.05, P < 0.01) decreases the AChE activity in both SS and DS portions
of brain tissue homogenate of rats as compared to disease control group.
4. Discussion
Natural nootropics are increasing in popularity owing to preference of the people [68]. Worldwide at present
there is an incredible urge to investigate medicinal plants for improving cognitive function in order to their less
adverse effects [69]. This is the first study showing neuroprotective activity of EEPR in aluminium-induced rats
of cognitive impairment and oxidative stress by using various behavioral and biochemical studies. In this study,
EEPR administration for 30 days showed significant neuroprotective effect by improving various types of mem-
ory, learning, antioxidant enzymes and anti-acetylcholinesterase activity in rats.
The PA test is commonly known as fear-aggravated test which is used to assess learning and memory [70]. In
PA test, rats learn to avoid an environment in which an aversive stimulus (i.e., foot-shock) was previously pro-
vided. In this test the measured parameters were ITL and STL. The latency times, once the rat had entered the
dark compartment was recorded as ITL as definite earlier. The STL is a measure of the memory of the aversive
experience [45]. The mean STL of rats treated with the EEPR were ominously higher than those of the other
groups. In the study of effect of Aronia melanocarpa fruits juice on memory in rats, Valcheva-Kuzmanova et al.,
also reported analogous findings [71]. The RA test is used to assess learning and memory based on alteration of
**
*
**
**
0
10
20
30
40
50
60
70
80
90
100
Con Alu Don Alu+EEPR
100
Alu+EEPR
200
Alu+Don
Memory Retention (%)
Treatment Groups
Md. S. Uddin et al.
96
Table 1. Nootropic Effect of EEPR on biochemical parameters of rat brain antioxidant defense system.
Treatment SOD (U/mg protein) Cat (U/min) TBARS (nM/min/mg protein)
Con 9.57 ± 1.62 10.25 ± 1.13 204.58 ± 4.03
Alu 6.89 ± 2.24 7.32 ± 2.05 294.76 ± 5.64
Don 26.57 ± 1.30 ** 23.98 ± 3.58 * 132.59 ± 4.37 *
Alu + EEPR 100 12.67 ± 2.96 * 11.04 ± 1.39 * 198.62 ± 6.79 *
Alu + EEPR 200 14.54 ± 3.45 * 14.58 ± 1.42 ** 182.98 ± 6.97 *
Alu + Don 18.95 ± 1.28 * 19.07 ± 2.71 ** 174.8 ± 5.74 **
The rats brain biochemical parameters were expressed as mean ± SEM values (n = 6/group). * P < 0.05, ** P < 0.01 significant difference from the dis-
ease control group.
Table 2. Nootropic Effect of EEPR on AChE activity in rat brain.
Treatment SS AChE (M/min/g protein) DS AChE (M/min/g protein)
Con 0.195 ± 0.052 0.772 ± 0.016
Alu 0.303 ± 0.013 1.216 ± 0.031
Don 0.085 ± 0.020 * 0.296 ± 0.027 *
Alu + EEPR 100 0.188 ± 0.042 * 0.812 ± 0.067 *
Alu + EEPR 200 0.153 ± 0.021 ** 0.595 ± 0.064 *
Alu + Don 0.106 ± 0.047 * 0.406 ± 0.076 **
The AChE activity for each group were expressed as mean ± SEM values (n = 6/group). * P < 0.05, ** P < 0.01 significant difference from the disease
control group.
the arms entry [72]. In RA test the measured parameters were WR and CR. Increased the number of CRs indi-
cated that the improvement of the learning and memory of rats. In the current study, improvement of learning
and memory was reported by EEPR. Sharma et al., in the study of neuroenhancing potentiality of Acacia auri-
culiformis leaves, monitored better learning and memory enhancing potentiality in rats [73].
Metabolism of oxygen is greatly responsible for producing superoxide and if not controlled causes many
types of cell damage [74]. SOD is a metalloenzyme that exert protection in cells exposed to oxygen. It catalyzes
the dismutation or partitioning of the superoxide (O 2− ·) radical into either ordinary molecular oxygen (O 2 ) or
hydrogen peroxide (H 2 O 2 ) [75]. Hydrogen peroxide is also harmful, but not more so and is degraded by other
enzymes including CAT. CAT is a haem-based enzyme in defensive the cell from oxidative damage by reactive
oxygen species (ROS) [76]. It catalyzes the transformation of H 2 O 2 to H 2 O and O 2 , thus shield cells from the
noxious effects of H 2 O 2 [77]. Study suggested that in each minute one molecule of CAT can convert approx-
imately 5 million H 2 O 2 to H 2 O and O 2 [78]. Presently it has become evident that the oxidation of lipids, or lipid
peroxidation, is a critical step in the pathogenesis of numerous disease states in all age’s patients [79]. Lipid pe-
roxidation is the by-product of oxidative degradation of lipids by the effect of various ROS (hydroxyl radical,
hydrogen peroxide etc.) [80]. It is the process in which free radicals steal electrons from the lipids in cell mem-
branes and finally causes cell damage. This process continues by a free radical chain reaction mechanism. It
most often affects PUFAs and initiating a self-propagating chain reaction [81]. Since lipid peroxidation is a
self-propagating chain-reaction, the initial oxidation of only a few lipid molecules can result in significant tissue
damage [82]. The destruction of membrane lipids and the end-products of such lipid peroxidation reactions are
especially dangerous for the viability of cells, even tissues [83]. The current study showed that administration of
EEPR pointedly increases the level of brain antioxidant enzymes and decrease the levels of TBARS. In the study
of nootropic activity of aerial parts of Persicaria flaccida on brain antioxidant markers and cognitive perfor-
mance of rats by Uddin et al., also reported equivalent results [84].
AChE is the major cholinesterase in the body [85]. It is an exzyme of carboxylesterase family that catalyzes
the breakdown of ACh and of some other choline esters that function as neurotransmitters. AChE is available in
primarily neuromuscular junctions and in chemical synapses of the cholinergic type [86]. The results of this
study exposed that AChE activity was significantly decreased in the EEPR treated rats. In the study of neuro-
Md. S. Uddin et al.
97
protective effect of Phyllanthus acidus fruits on learning and memory impairment in scopolamine-induced ani-
mal model of dementia and oxidative stress, Uddin et al., disclosed increases brain acetylcholine levels and en-
hances cognitive function in rats [87].
The consequence of this study suggested that administration of EEPR for 30 days produced superior nootropic
effect and reversed aluminium-induced cognitive dysfunction and oxidative stress in rats.
5. Conclusion
This study concludes that EEPR fruit has a potential nootropic effect on altering the aluminium-induced cogni-
tive dysfunction and oxidative stress in rats brain by improving cognitive functions, brain antioxidant enzymes
and anti-acetylcholinesterase activity. Therefore, this fruit extract can be used in controlling neurodegenerative
diseases more precisely AD. Despite these outcomes, further studies are required for isolation and identification
of promising nootropic compound(s) and disclose the possible mechanism of action.
Acknowledgements
The authors wish to thank the Department of Pharmacy, Southeast University, Dhaka, Bangladesh for providing
research facilities.
Ethical Approval
The study protocol was approved by the ethics committee of the Department of Pharmacy, Southeast University,
Dhaka, Bangladesh. The care and use of the animals were followed in accordance with the principles of NIH.
Authors’ Contributions
This work was carried out in collaboration among all authors. MSU designed the study, wrote the protocol and
managed the analyses of the study. MSU, AAM and MAI performed the laboratory experiments and prepared
the draft of the manuscript. AI and MFH prepared the plant extract and performed literature review. SK per-
formed the statistical analysis. MR reviewed the scientific contents of the manuscript. All authors read and ap-
proved the final manuscript.
Competing Interests
The authors proclaim that they have no competing interests.
References
[1] Rouch, S., Dorey, J.-M., Boublay, N., Henaff, M.-A., Dibie-Racoupeau, F., Makaroff, Z., et al. (2014) Personality,
Alzheimer’s Disease and Behavioural and Cognitive Symptoms of Dementia: The PACO Prospective Cohort Study
Protocol. BMC Geriatrics, 14, 1-10.
[2] Gaugler, J.E., Ascher-Svanum, H., Roth, D.L., Fafowora, T., Siderowf, A. and Beach, T.G. (2013) Characteristics of
Patients Misdiagnosed with Alzheimer’s Disease and their Medication Use: An Analysis of the NACC-UDS Database.
BMC Geriatrics, 13, 1-10.
http://dx.doi.org/10.1186/1471-2318-13-137[3] Serrano-Pozo, A., Frosch, M.P., Masliah, E. and Hyman, B.T. (2011) Neuropathological Alterations in Alzheimer
Disease. Cold Spring Harbor Perspectives in Medicine, 1, Article ID: a006189.
http://dx.doi.org/10.1101/cshperspect.a006189[4] McGleenon, B.M., Dynan, K.B. and Passmore, A.P. (1999) Acetylcholinesterase Inhibitors in Alzheimer’s Disease.
British Journal of Clinical Pharmacology, 48, 471-480.
http://dx.doi.org/10.1046/j.1365-2125.1999.00026.x[5] Francis P.T., Palmer, A.M., Snape, M. and Wilcock, G.K. (1999) The Cholinergic Hypothesis of Alzheimer’s Disease:
A Review of Progress. Journal of Neurology, Neurosurgery & Psychiatry, 66, 137-147.
http://dx.doi.org/10.1136/jnnp.66.2.137[6] Goverdhan, P., Sravanthi, A. and Mamatha, T. (2012) Neuroprotective Effects of Meloxicam and Selegiline in Scopo-
lamine-Induced Cognitive Impairment and Oxidative Stress. International Journal of Alzheimer’s Disease, 2012, 1-7.
http://dx.doi.org/10.1155/2012/974013[7] Hazzan, A.A., Ploeg, J., Shannon, H., Raina, P. and Oremus, M. (2015) Caregiver Perceptions Regarding the Mea-
surement of Level and Quality of Care in Alzheimer’s Disease. BMC Nursing, 14, 1-7.
Md. S. Uddin et al.
98
[8] Yang, H.-Q., Sun, Z.-K. and Chen, S.-D. (2012) Current Advances in the Treatment of Alzheimer’s Disease: Focused
on Considerations Targeting Aβ and Tau. Translational Neurodegeneration, 1, 1-10.
http://dx.doi.org/10.1186/2047-9158-1-21[9] Bindu, A.H., Siddiqui, A. and Jeevani, T. (2011) Genetic and Degenerative Neurological Disorders—An Emphasis on
Alzheimer’s, the Mystery. Journal of Genetic Syndromes & Gene Therapy, 2, 1-4.
[10] Lobo, V., Patil, A., Phatak, A. and Chandra, N. (2010) Free Radicals, Antioxidants and Functional Foods: Impact on
Human Health. Pharmacognosy Reviews, 4, 118-126.
http://dx.doi.org/10.4103/0973-7847.70902[11] Markesbery, W.R. (1999) The Role of Oxidative Stress in Alzheimer Disease. Archives of Neurology, 56, 1449-1452.
http://dx.doi.org/10.1001/archneur.56.12.1449[12] Rahal, A., Kumar, A., Singh, V., Yadav, B., Tiwari, R., Chakraborty, S., et al. (2014) Oxidative Stress, Prooxidants,
and Antioxidants: The Interplay. BioMed Research International, 1-12.
http://dx.doi.org/10.1155/2014/761264[13] Zhao, Y. and Zhao, B. (2013) Oxidative Stress and the Pathogenesis of Alzheimer’s Disease. Oxidative Medicine and
Cellular Longevity, 1-9.
http://dx.doi.org/10.1155/2013/316523[14] Cui, H., Kong, Y. and Zhang, H. (2012) Oxidative Stress, Mitochondrial Dysfunction, and Aging. Journal of Signal
Transduction, 1-6.
http://dx.doi.org/10.1155/2012/646354[15] Perry, G., Cash, A.D., Mark, A. and Smith, M.A. (2002) Alzheimer Disease and Oxidative Stress. Journal of Biomedi-
cine and Biotechnology, 2, 120-123.
http://dx.doi.org/10.1155/S1110724302203010[16] Feng, Y. and Wang, X. (2012) Antioxidant Therapies for Alzheimer’s Disease. Oxidative Medicine and Cellular Lon-
gevity, 2012, Article ID: 472932.
http://dx.doi.org/10.1155/2012/472932[17] Lovell, M.A. and Markesbery, W.R. (2007) Oxidative DNA Damage in Mild Cognitive Impairment and Late-Stage
Alzheimer’s Disease. Nucleic Acids Research, 35, 7497-7504.
http://dx.doi.org/10.1093/nar/gkm821[18] Hamilton, R.T., Bhattacharya, A., Walsh, M.E., Shi, Y., Wei, R., Zhang, Y., et al. (2013) Elevated Protein Carbonyla-
tion, and Misfolding in Sciatic Nerve from db/db and Sod1 −/− Mice: Plausible Link between Oxidative Stress and De-
myelination. PLoS ONE, 8, e65725.
http://dx.doi.org/10.1371/journal.pone.0065725[19] Nuss, J.E., Amaning, J.K., Bailey, C.E., DeFord, J.H., Dimayuga, V.L., Rabek, J.P., et al. (2009) Oxidative Modifica-
tion and Aggregation of Creatine Kinase from Aged Mouse Skeletal Muscle. Aging, 1, 557-572.
http://dx.doi.org/10.18632/aging.100055[20] Huang, W.-J., Zhang, X. and Chen, W.-W. (2016) Role of Oxidative Stress in Alzheimer’s Disease. Biomedical Re-
ports, 4, 519-522.
http://dx.doi.org/10.3892/br.2016.630[21] Ayala, A., Muñoz, M.F. and Argüelles, S. (2014) Lipid Peroxidation: Production, Metabolism, and Signaling Mechan-
isms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Medicine and Cellular Longevity, 2014, Article ID:
360438.
http://dx.doi.org/10.1155/2014/360438[22] Ozias, M.K., Carlson, S.E. and Levant, B. (2007) Maternal Parity and Diet (n-3) Polyunsaturated Fatty Acid Concen-
tration Influence Accretion of Brain Phospholipid Docosahexaenoic Acid in Developing Rats. Journal of Nutrition,
137, 125-129.
[23] Milkovic, L., Gasparovic, A.C. and Zarkovic, N. (2015) Overview on Major Lipid Peroxidation Bioactive Factor 4-
Hydroxynonenal as Pluripotent Growth-Regulating Factor. Free Radical Research, 49, 850-860.
http://dx.doi.org/10.3109/10715762.2014.999056[24] Leonora, J.L., Hoehr, N., Mazur, S.J., Dianov, G.L., Sentürker, S., Dizdaroglu, M., et al. (1999) Repair of Oxidative
DNA Base Lesions Induced by Fluorescent Light Is Defective in Xeroderma Pigmentosum Group A Cells. Nucleic
Acids Research, 27, 3153-3158.
http://dx.doi.org/10.1093/nar/27.15.3153[25] Rahman, K. (2007) Studies on Free Radicals, Antioxidants, and Co-Factors. Clinical Interventions in Aging, 2, 219-
236.
[26] Shao, C., Xiong, S., Li, G.-M., Gu, L., Mao, G. and Markesbery, W.R. (2008) Altered 8-Oxoguanine Glycosylase in
Mild Cognitive Impairment and Late-Stage Alzheimer’s Disease Brain. Free Radical Biology & Medicine, 45, 813-819.
http://dx.doi.org/10.1016/j.freeradbiomed.2008.06.003[27] Gella, A. and Durany, N. (2009) Oxidative Stress in Alzheimer Disease. Cell Adhesion & Migration, 3, 88-93.
http://dx.doi.org/10.4161/cam.3.1.7402[28] Gandhi, S. and Abramov, A.Y. (2012) Mechanism of Oxidative Stress in Neurodegeneration. Oxidative Medicine and
Cellular Longevity, 2012, Article ID: 428010.
http://dx.doi.org/10.1155/2012/428010[29] Miu, A.C. and Benga, O. (2006) Aluminum and Alzheimer’s Disease: A New Look. Journal of Alzheimer’s Disease,
10, 179-201.
[30] Exley, C. (2001) Aluminum and Alzheimer’s Disease. Journal of Alzheimer’s Disease, 3, 551-552.
[31] Banks, W.A. and Kastin, A.J. (1985) Aluminum Alters the Permeability of the Blood-Brain Barrier to Some Non-Pep-
Md. S. Uddin et al.
99
tides. Neuropharmacology, 24, 407-412.
http://dx.doi.org/10.1016/0028-3908(85)90025-5[32] Struys-Ponsar, C., Kerkhofs, A., Gauthier, A., Soffie, M. and Van den Bosch de Aguilar, P. (1997) Effects of Alumi-
num Exposure on Behavioral Parameters in the Rat. Pharmacology Biochemistry and Behavior, 56, 643-648.
http://dx.doi.org/10.1016/S0091-3057(96)00515-1[33] Platt, B., Fiddler, G., Riedel, G. and Henderson, Z. (2001) Aluminium Toxicity in the Rat Brain: Histochemical and
Immunocytochemical Evidence. Brain Research Bulletin, 55, 257-267.
http://dx.doi.org/10.1016/S0361-9230(01)00511-1[34] Kawahara, M., Kato, M. and Kuroda, Y. (2001) Effects of Aluminum on the Neurotoxicity of Primary Cultured Neu-
rons and on the Aggregation of Beta-Amyloid Protein. Brain Research Bulletin, 55, 211-217.
http://dx.doi.org/10.1016/S0361-9230(01)00475-0[35] Kawahara, M. (2005) Effects of Aluminum on the Nervous System and Its Possible Link with Neurodegenerative Dis-
eases. Journal of Alzheimer’s Disease, 8, 171-182.
[36] Johnson, G.V. and Jope, R.S. (1986) Aluminum Impairs Glucose Utilization and Cholinergic Activity in Rat Brain in
Vitro. Toxicology, 40, 93-102.
http://dx.doi.org/10.1016/0300-483X(86)90049-1[37] Bondy, S.C., Guo-Ross, S.X. and Pien, J. (1998) Mechanisms Underlying the Aluminum-Induced Potentiation of the
Pro-Oxidant Properties of Transition Metals. Neurotoxicology, 19, 65-71.
[38] Pham-Huy, L.A., He, H. and Pham-Huy, C. (2008) Free Radicals, Antioxidants in Disease and Health. International
Journal of Biomedical Science, 4, 89-96.
[39] Satyanarayana, U., Kumar, A.N., Naidu, J.N. and Prasad, D.K.V. (2014) Antioxidant Supplementation for Health—A
Boon or a Bane? Journal of Dr. NTR University of Health Sciences, 3, 221-230.
http://dx.doi.org/10.4103/2277-8632.146595[40] Chen, L.-E., Wu, F., Zhao, A., Ge, H. and Zhan, H. (2016) Protection Efficacy of the Extract of Ginkgo biloba against
the Learning and Memory Damage of Rats under Repeated High Sustained +Gz Exposure. Evidence-Based Comple-
mentary and Alternative Medicine, 2016, Article ID: 6320586.
[41] Aguiar, S. and Borowski, T. (2013) Neuropharmacological Review of the Nootropic Herb Bacopa monnieri. Rejuvena-
tion Research, 16, 313-326.
http://dx.doi.org/10.1089/rej.2013.1431[42] Singhal, A.K., Naithani, V. and Bangar, O.P. (2012) Medicinal Plants with a Potential to Treat Alzheimer and Asso-
ciated Symptoms. International Journal of Nutrition, Pharmacology, Neurological Diseases, 2, 84-91.
http://dx.doi.org/10.4103/2231-0738.95927[43] Shalini, S. and Sunil, K. (2013) Phyllanthus reticulatus Poir—An Important Medicinal Plant: A Review of Its Phyto-
chemistry, Traditional Uses and Pharmacological Properties. International Journal of Pharmaceutical Sciences & Re-
search, 4, 2528.
[44] Shruthi, S.D., Ramachandra, Y.L., Rai, S.P. and Jha, P.K. (2010) Pharmacognostic Evaluation of the Leaves of Kirga-
nelia reticulata Baill. (Euphorbiaceae). Asian and Australasian Journal of Plant Science and Biotechnology, 4, 62-65.
[45] Mandisa Kondlo Walter Sisulu National Botanical Garden (2010) Phyllanthus reticulatus Poir.
http://www.plantzafrica.com/plantnop/phyllanthusret.htm[46] Shalini, S., Sunil, K., Kumar, S., Sharma, S., Kumar, D., Kumar, T., et al. (2012) Pharmacognostic Study and An-
ti-Inflammatory Activity of Phyllanthus reticulatus Poir. Fruit. Asian Pacific Journal of Tropical Disease, 2, S332-
S335.
[47] Jamal, A.K., Yaacob, W.A. and Din, L.B. (2008) A Chemical Study on Phyllanthus reticulatus. Journal of Physical
Science, 19, 45-50.
[48] Begum, T., Rahman, S.M. and Rashid, A.M. (2006) Phytochemical and Biological Investigations of Phyllanthus reti-
culatus. Dhaka University Journal of Pharmaceutical Sciences, 5, 21-23.
[49] Aswatha Ram, H.N., Shreedhara, C.S., Gajera, F.P. and Zanwar, S.B. (2008) In Vitro Free Radical Scavenging Poten-
tial of Methanol Extract of Entire Plant of Phyllanthus reticulates Poir. Pharmacologyonline, 2, 440-451.
[50] National Research Council (2011) Guide for the Care and Use of Laboratory Animals. National Academies Press,
Washington DC.
[51] Weon, J.B., Lee, J., Eom, M.R., Jung, Y.S. and Ma, C.J. (2014) The Effects of Loranthus parasiticus on Scopola-
mine-Induced Memory Impairment in Mice. Journal of Evidence-Based Complementary & Alternative Medicine, 2014,
Article ID: 860180.
[52] Nannepaga, J.S., Korivi, M., Tirumanyam, M., Bommavaram, M. and Kuo, C.-H. (2014) Neuroprotective Effects of
Bacopa monniera Whole-Plant Extract against Aluminum-Induced Hippocampus Damage in Rats: Evidence from
Electron Microscopic Images. The Chinese Journal of Physiology, 57, 279-285.
http://dx.doi.org/10.4077/CJP.2014.BAC221Md. S. Uddin et al.
100
[53] Organization for Economic cooperation and Development (2002) OECD Guidelines for the Testing of Chemicals:
Acute Oral Toxicity—Acute Toxic Class Method. OECD Environment, Health and Safety Publications, Paris.
[54] Akar, F., Mutlu, O., Celikyurt, I.K., Ulak, G., Erden, F., Bektas, E. and Tanyeri, P. (2014) Zaprinast and Rolipram En-
hances Spatial and Emotional Memory in the Elevated Plus Maze and Passive Avoidance Tests and Diminishes Explo-
ratory Activity in Naive Mice. Medical Science Monitor Basic Research, 20, 105-111.
http://dx.doi.org/10.12659/MSMBR.891149[55] Van der Staay, F.J., Schuurman, T., van Reenen, C.G. and Korte, S.M. (2009) Emotional Reactivity and Cognitive
Performance in Aversively Motivated Tasks: A Comparison between Four Rat Strains. Behavioral and Brain Func-
tions, 5, 50.
http://dx.doi.org/10.1186/1744-9081-5-50[56] Wang, J., Wang, X., Lv, B., Yuan, W., Feng, Z., Weidong, M.I., et al. (2014) Effects of Fructus akebiae on Learning
and Memory Impairment in a Scopolamine-Induced Animal Model of Dementia. Experimental and Therapeutic Medi-
cine, 8, 671-675.
http://dx.doi.org/10.3892/etm.2014.1775[57] Saadipour, K., Sarkaki, A., Alaei, H., Badavi, M. and Rahim, F. (2009) Forced Exercise Improves Passive Avoidance
Memory in Morphine-Exposed Rats. Pakistan Journal of Biological Sciences, 12, 1206-1211.
http://dx.doi.org/10.3923/pjbs.2009.1206.1211[58] Benchenane, K., Castel, H., Boulouard, M., Bluthe, R., Fernandez-Monreal, M., Roussel, B.D., et al. (2007) Anti-NR1
N-Terminal-Domain Vaccination Unmasks the Crucial Action of tPA on NMDA-Receptor-Mediated Toxicity and
Spatial Memory. Journal of Cell Science, 120, 578-585.
http://dx.doi.org/10.1242/jcs.03354[59] Sossin, W.S., Lacaille, J.-C., Castellucci, V.F. and Belleville, S. (2008) Progress in Brain Research: Essence of Mem-
ory. Elsevier, Amsterdam.
[60] Rao, M.K., Rao, M.S. and Rao, G.S. (2007) Treatment with Centella asiatica (Linn) Fresh Leaf Extract Enhances
Learning Ability and Memory Retention Power in Rats. Neurosciences, 12, 236-241.
[61] Deacon, R.M. and Rawlins, J.N. (2006) T-Maze Alternation in the Rodent. Nature Protocols, 1, 7-12.
http://dx.doi.org/10.1038/nprot.2006.2[62] Morris, R. (1984) Developments of a Water-Maze Procedure for Studying Spatial Learning in the Rat. Journal of
Neuroscience Methods, 11, 47-60.
http://dx.doi.org/10.1016/0165-0270(84)90007-4[63] Kameyama, T., Nabeshima, T. and Kozawa, T. (1986) Step-down-Type Passive Avoidance- and Escape-Learning Me-
thod: Suitability for Experimental Amnesia Models. Journal of Pharmacological Methods, 16, 39-52.
http://dx.doi.org/10.1016/0160-5402(86)90027-6[64] Bhaskar, M. and Chintamaneni, M. (2014) Investigating the role of Eclipta alba on Brain Antioxidant Markers, Cogni-
tive Performance and Acetylcholinesterase Activity of Rats. International Journal of Pharmaceutical and Phytophar-
macological Research (eIJPPR), 3, 390-394.
[65] Chance, B. and Maehly, A.C. (1955) Assay of Catalase and Peroxidases. Methods in Enzymology, 11, 764-775.
http://dx.doi.org/10.1016/S0076-6879(55)02300-8[66] Iqbal, M., Sharma, M.D., Zadeh, H.R., Hasan, N., Abdulla, M., Athar, M., et al. (1996) Glutathione Metabolizing En-
zymes and Oxidative Stress in Ferric Nitrilotriacetate (Fe-NTA) Mediated Hepatic Injury. Redox Report, 2, 385-391.
[67] Ellman, G.L., Courtney, K.D., Andres, V. and Featherstone, R.M. (1961) A New and Rapid Colorimetric Determina-
tion of Acetylcholinesterase Activity. Biochemical Pharmacology, 7, 88-95.
http://dx.doi.org/10.1016/0006-2952(61)90145-9[68] Kumar, G.P. and Khanum, F. (2012) Neuroprotective Potential of Phytochemicals. Pharmacognosy Reviews, 6, 81-90.
http://dx.doi.org/10.4103/0973-7847.99898[69] Kulkarni, R., Girish, K.J. and Kumar, A. (2012) Nootropic Herbs (Medhya Rasayana) in Ayurveda: An Update. Phar-
macognosy Reviews, 6, 147-153.
http://dx.doi.org/10.4103/0973-7847.99949[70] Ogren, S.O., Stone, W.S. and Altman, H.J. (1987) Evidence for a Functional Interaction between Serotonergic and
Cholinergic Mechanisms in Memory Retrieval. Behavioral and Neural Biology, 48, 49-62.
http://dx.doi.org/10.1016/S0163-1047(87)90574-7[71] Valcheva-Kuzmanova, S.V., Eftimov, M.T., Tashev, R.E., Belcheva, I.P. and Belcheva, S.P. (2014) Memory Effects of
Aronia melanocarpa Fruit Juice in a Passive Avoidance Test in Rats. Folia Medica, 56, 199-203.
http://dx.doi.org/10.2478/folmed-2014-0029[72] Savage, L.M., Hall, J.M. and Vetreno, R.P. (2011) Anterior Thalamic Lesions Alter both Hippocampal-Dependent
Behavior and Hippocampal Acetylcholine Release in the Rat. Learning & Memory, 18, 751-758.
http://dx.doi.org/10.1101/lm.023887.111[73] Sharma, A., Shetty, M., Parida, A., Adiga, S., Kamath, S. and Sowjanya (2014) Effect of Ethanolic Extract of Acacia
auriculiformis Leaves on Learning and Memory in Rats. Pharmacognosy Research, 6, 246-250.
Md. S. Uddin et al.
101
http://dx.doi.org/10.4103/0974-8490.132605[74] Salin, M. and McCord, J. (1975) Free Radicals and Inflammation. Protection of Phagocytosing Leukocytes by Supe-
roxide Dismutase. The Journal of Clinical Investigation, 56, 1319-1323.
http://dx.doi.org/10.1172/JCI108208[75] Ansari, M.A. and Scheff, S.W. (2010) Oxidative Stress in the Progression of Alzheimer Disease in the Frontal Cortex.
Journal of Neuropathology & Experimental Neurology, 69, 155-167.
http://dx.doi.org/10.1097/NEN.0b013e3181cb5af4[76] Sharma, P., Jha, A.B., Dubey, R.S. and Pessarakli, M. (2012) Reactive Oxygen Species, Oxidative Damage, and Anti-
oxidative Defense Mechanism in Plants under Stressful Conditions. Journal of Botany, 2012, Article ID: 217037.
http://dx.doi.org/10.1155/2012/217037[77] Spitz, D.R., Adams, D.T., Sherman, C.M. and Roberts, R.J. (1992) Mechanisms of Cellular Resistance to Hydrogen
Peroxide, Hyperoxia, and 4-Hydroxy-2-nonenal Toxicity: The Significance of Increased Catalase Activity in H 2 O 2 -Re-
sistant Fibroblasts. Archives of Biochemistry and Biophysics, 292, 221-227.
http://dx.doi.org/10.1016/0003-9861(92)90071-4[78] Aoyama, K. and Nakaki, T. (2015) Glutathione in Cellular Redox Homeostasis: Association with the Excitatory Amino
Acid Carrier 1 (EAAC1). Molecules, 20, 8742-8758.
http://dx.doi.org/10.3390/molecules20058742[79] Gammone, M.A., Riccioni, G. and D’Orazio, N. (2015) Marine Carotenoids against Oxidative Stress: Effects on Hu-
man Health. Marine Drugs, 13, 6226-6246.
http://dx.doi.org/10.3390/md13106226[80] Powers, S.K. and Jackson, M.J. (2008) Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle
Force Production. Physiological Reviews, 88, 1243-1276.
http://dx.doi.org/10.1152/physrev.00031.2007[81] Madhura, T.K. (2015) Role of Oxidative Stress in the Pathogenesis of OCD. Biochemistry & Analytical Biochemistry,
4, 217.
[82] Sosa, R.A., Murphey, C., Robinson, R.R. and Forsthuber, T.G. (2015) IFN-γ Ameliorates Autoimmune Encephalo-
myelitis by Limiting Myelin Lipid Peroxidation. Proceedings of the National Academy of Sciences of the United States
of America, 112, E5038-E5047.
[83] Mylonas, C. and Kouretas, D. (1999) Lipid Peroxidation and Tissue Damage. In Vivo, 13, 295-309.
[84] Uddin, M.S., Nasrullah, M., Hossain, M.S., Rahman, M.M., Sarwar, M.S., Amran, M.S., et al. (2016) Evaluation of
Nootropic Activity of Persicaria flaccida on Cognitive Performance, Brain Antioxidant Markers and Acetylcholines-
terase Activity in Rats: Implication for the Management of Alzheimer’s Disease. American Journal of Psychiatry and
Neuroscience, 4, 26-37.
http://dx.doi.org/10.11648/j.ajpn.20160402.12[85] Čolović, M.B., Krstić, D.Z., Lazarević-Pašti, T.D., Bondžić, A.M. and Vasić, V.M. (2013) Acetylcholinesterase Inhi-
bitors: Pharmacology and Toxicology. Current Neuropharmacology, 11, 315-335.
http://dx.doi.org/10.2174/1570159X11311030006[86] Oren, M., Brikner, I., Appelbaum, L. and Levy, O. (2014) Fast Neurotransmission Related Genes Are Expressed in
Non Nervous Endoderm in the Sea Anemone Nematostella vectensis. PLoS ONE, 9, e93832.
http://dx.doi.org/10.1371/journal.pone.0093832[87] Uddin, M.S., Mamun, A.A., Hossain, M.S., Ashaduzzaman, M., Noor, M.A.A., Hossain, M.S., Uddin, M.J., Sarker, J.
and Asaduzzaman, M. (2016) Neuroprotective Effect of Phyllanthus acidus L. on Learning and Memory Impairment in
Scopolamine-Induced Animal Model of Dementia and Oxidative Stress: Natural Wonder for Regulating the Develop

 Analyzing Nootropic Effect of Phyllanthus
Analyzing Nootropic Effect of Phyllanthus