ABSTRACT The purpose of our investigations
was to measure, in a co-culture condition, the immunoresponse to allogeneic or
xenogenic cells, selected as potential sources for cell therapy of arthritis.
We challenged human spleen-derived cells (hSpl) by three different mechanisms:
1) exposure to donor allogeneic or xenogeneic cellular antigens; 2) exposure to
donor cells transduced with adenoviral antigens (Ad) and 3) lipopolysaccharide
(LPS), a known inflammatory immunostimulant. The immunoresponse to allogeneic
human synovial-derived mesenchymal stromal cells alone or transduced with
adenoviral green fluorescent protein (hSD-MSC or hSD-MSC/GFP) or the
immunoresponse to xenogeneic equine mesenchymal stromal cells (eqMSC) or equine
dermal fibroblasts (eqDFb), characterized by the proportion of CD3+ , CD4+ ,
and CD8+ human splenocytes (hSpl), was measured on Day 0 and Day 6 of co-culture
by flow cytometry. In culture with hSD-MSC, hSD-MSC/GFP, eqDFb, or eqMSC, the
proportion of CD3+ and CD8+ hSpl increased with time in culture but not with
exposure to cell allo- or xeno-antigens. Both hSD-MSC and hSD-MSC/GFP increased
in number during culture and were not affected in viability or proliferation by
co-culture with allogeneic hSpl. In this in vitro, primary exposure study, hSpl
demonstrated a natural selection and adaptation to a short-term cell culture
environment, and that neither allogeneic nor xenogeneic cell antigens incited a
greater cellular immunoactivation than co-cultured hSpl alone. Keywords:
Arthritis; Stem Cells; Allogeneic; Xenogeneic; Immunoresponse 1. Introduction
Cutting-edge advancements in regenerative medicine may harness the potential of
an engineered cell source as a therapeutic vector for the repair and
restoration of multiple human tissues including articular cartilage damaged by
injury or degenerated through either osteoarthritis or rheumatoid arthritis.
Allogeneic cells, from a different organism of the same species or xenogenic
cells, from a different species, could provide a nearly limitless supply of
therapeutic cells for use in tissue repair. Allogeneic or xenogeneic cell
sources, engineered to serve as a clinical tool, could dramatically and
irrevocably enhance current medical practices that promote healing. Allogeneic
mesenchymal stem cells in Phase III clinical trials, have been used as a
treatment for inflammatory conditions including acute Bone Versus Graft Disease
and Crohn’s disease [1]. In orthopaedic medicine, autologus cell-based
strategies for tissue repair and restoration, including autologous chondroctye
implantation and osteochondral grafts, are currently used in clinical practice
with some promising results [2-6]. A potentially promising alternative or
adjunct strategy to current regenerative techniques could use allogeneic cells
or xenogenic cells. To this end, a cell-based allogeneic therapy has been
provided for 1st generation, commercially-available cartilage neograft (DeNovo®
ET Live Chondral Engineered Tissue Graft, Zimmer Holdings, Inc). This product,
approved last year, relies on allogeneic, juvenile chondrocytes and a
proprietary cell-scaffold system to promote healing. Potentially, allogeneic or
xenogeneic cells could serve as effective therapeutic vectors in vivo to
integrate into the native biological environment of tissue. The immunoresponse
of allogeneic and xenogeneic cells needs to be further investigated, and
further observations of the cellular mechanisms and interactions will help to
elucidate and describe the immunoresponse for future clinical trials. New cell
sources could improve the repair and restoration of articular cartilage, such
as in injured cartilage or deteriorating cartilage as in osteoarthritis (OA),
the most common form of arthritis. It is believed that there are immune
processes that could be responsible for the deCopyright © 2012 SciRes. OJCB 2
S. S. JUMP ET AL. generation of cartilage. In particular, activated immune cell
infiltration, including T-cells, has been associated with the advancement of
arthritis [7-9]. Finding a potential cell- or tissue-based treatment for
damaged cartilage is dependent upon further understanding the immunoresponse of
cells from an articular joint, including synovial lining cells or chondrocytes.
Organ or cell transplantation is characterized by an activation of host
defenses including the activation and proliferation of immune cell types
including cluster determination 3 (CD3+ ) mature lymphocytes, cluster
determination 4 (CD4+ ) T-helper (TH1) cells, and cluster determination (CD8+ )
natural killer/ cytotoxic T lymphocytes (CTL) [7-9]. Allogeneic
synovial-derived cells (SDSCs) have been successful in the repair of
full-thickness defects of the femoral condyle in rats; however, contaminating
macrophages provided evidence of a delayed immune reaction to the
transplantated allogeneic cells [10]. Furthermore, xenogeneic SDSCs failed to
repair cartilage defects in vivo, and an enhanced immune response,
characterized by detection of major histocompatability complex antigen II
(MHCII) in foreign bodies found in the repair tissue [11]. In cell culture, a
limited number of studies have used a mixed immune cell design to evaluate host
versus donor reactions [12-16]. Allogeneic human MSCs derived from bone marrow
successfully reduced CD8+ expansion in cell culture providing support for the
beneficial immunomodulation of MSCs [17], and allogeneic fibroblast-like
synoviocytes have also reduced proliferation in T-cells found in bone marrow
[12]. On the other hand, allogeneic peripheral blood mononuclear cells elicited
an activation of both CD4+ and CD8+ cells [14]. Further characterization of the
potential cellular activation of allogeneic or xenogeneic cells with the host
immune system is needed to further develop clinical tools to control or monitor
this reaction for the future of cell therapy as a treatment for joint disease.
Our study will extend the findings in the literature by using another source of
human immune cells, splenic tissue, to investigate the potential immune
activation of CD3+ , CD4+ , and CD8+ cell types to allogeneic or xenogeneic
mesenchymal stromal cells. The purpose of our investigations was to measure, in
a co-culture condition, the immunoresponse to allogeneic or xenogenic cells,
selected as potential sources for cell therapy of arthritis. We challenged
human spleen-derived cells (hSpl) by three different mechanisms: 1) exposure to
donor allogeneic or xenogeneic cellular antigens; 2) exposure to donor cells
transduced with adenoviral antigens (Ad); and 3) lipopolysaccharide (LPS), a
known inflammatory immunostimulant. For the allogeneic experiment, human
synovial-derived mesenchymal stromal cells (hSC-MSC) or hSC-MSC transduced with
adenovirus expressing green fluorescent protein (hSC-MSCGFP) were co-cultured
with allogeneic hSpl. In the xenogeneic experiment, equine (eq) bone-marrow
derived MSCs (eqMSC) or equine dermal fibroblasts (eqDFb) were co-cultured with
hSpl. Our hypothesis was that a cell-mediated challenge of either allogeneic or
xenogeneic cells would stimulate the formation and development of CD3+ and CD8+
hSpl in co-culture compared to unchallenged hSpl and that adenoviral challenge
may further enhance this effect. Additionally, the viability of the allogeneic
or xenogeneic donor cells was expected to be reduced when co-cultured with
human host cells. 2. Methods 2.1. Human and Equine Donor Tissue Harvest and
Digestion Synovial biopsies were obtained from the knee joint of orthopedic
patients undergoing anterior cruciate ligament reconstruction by an author
surgeon (DCF). Tissue harvest was conducted in accordance with the guidelines
set by the Institutional Review Board (IRB Protocol 2009H0256) at The Ohio
State University and only by consent of the patient. Synovial biopsies were
digested in sterile-filtered (0.2 um) media containing collagenase type I (0.2%
m/v) for 90 min (Gibco, Carlsbad, CA). Following digestion, cells were filtered
through a cell strainer (70 μM) and washed twice in Gey’s Balanced Salt (GBSS)
(Sigma-Aldrich, St. Louis). Before initial seeding, cell samples from synovium
were exposed to trypan blue exclusion stain and cell count and cell viability
was determined using a hemacytometer Bone marrow aspirates were obtained from
the sternum of adult horses immediately after euthanasia for reasons unrelated
to the immune system. A bone marrow aspiration needle (MD Tech Inc,
Gainesville, FL) was inserted into a sternebral body from the ventral aspect of
the sternum, and marrow was aspirated into a sterile, heparin-flushed (American
Pharmaceutical Partners Inc., Schaumburg, IL), 12-mL syringe. The procedure was
repeated until a minimum of 10 mL of bone marrow was collected. Primary
BMD-MSCs were isolated via centrifugation of marrow specimens and cultured in a
monolayer, as has been described [18]. Derived eqBMD-MSCs (eqMSC) were
confirmed as pluripotent by culturing in controlled osteogenic, chondrogenic,
and adipogenic media containing dexamethasone with ascorbate, recombinant human
transforming growth factor-I, and dex methasone with insulin and indomethacin
(Gibco, Grand Island, NY) [19-21]. Dermal fibroblasts (DFb) were obtained via
skin biopsy as part of another equine study [20,22]. Full-thickness skin tissue
was harvested using a 5 mm diameter biopsy punch from the pectoral region (10 -
12 punches Copyright © 2012 SciRes. OJCB S. S. JUMP ET AL. 3 per horse) from
each of six adult horses. The dermal layer was dissected from the epidermis
under a microscope, and DFbs were isolated by type-1 collagenase digestion
(GIBCO, Grand Island, NY) and cultured in DMEM supplemented with L-glutamine
(300 mg/mL), penicillin (30 mg/mL), streptomycin (30 mg/mL), and 10% fetal
bovine serum at 37˚C in a 5% CO2 atmosphere. Synovial-derived mesenchymal
stromal cells were passaged a minimum of 4 times, but no more than 7 times.
Previous studies in the literature have demonstrated that hSD-MSCs cells
maintain a consistent phenotype between passages 3 and 8 [23,24]. Equine BMD- MSC
and Dfb were low passage (<3 passages). 2.2. Tissue Harvest of Host Mixed Immune Cells Human spleens were selected as the host mixed cell population for co-culture experiments. Approval for receiving portions of human spleens was granted by Lifeline of Ohio, an organ donor center in Columbus, Ohio. Splenocytes were harvested and prepared with high yield and successfully grown in cell culture using methods adapted from murine splenocyte culture [25,26]. Briefly, tissue was trimmed into small pieces and thoroughly minced using a syringe plunger inside of a nylon cell strainer (70 μm) in a 35 mm cell culture plate. Cells were digested using Ack lysing buffer (Gibco, Carlsbad, CA), and the samples were subjected to consecutive washes in ice cold PBS. Isolated splenocytes were immediately allocated to cultures as described below in experimental design. 2.3. Adenoviral Transduction of Donor Allogeneic Synovial-Derived Mesenchymal Stromal Cells Recombinant, E1-deficient, serotype-5 adenovirus vectors containing the open reading frame segment of human GFP (AdGFP) under the control of the cytomegalovirus promoter were generated. Successful transduction of AdGFP was verified in cell culture. Viral titer [infection units per mL, (IFU/mL)] was determined (Adeno-X Rapid TiterKit; Clonetech, Mountain View, CA, USA) and SD-MSCs were transduced at 100 multiplicities of infection (MOI) or 1 × 102 infectious units per cell [19- 21]. 2.4. Cell Co-Culture Conditions and Experimental Design For the overall experimental design, three sources of donor cells, hSD-MSC (allogeneic; Experiment 1) and eqMSC and eqDFb (xenogeneic; Experiment 2), were seeded at 10,000 cells/cm2 onto 24-well cell culture plates at Day –2 in 10% FBS containing 1% penicillin streptomycin in DMEM (Invitrogen). Human SD-MSC cultures were transduced with Ad-GFP on Day –1. For Experiments 1 and 2, isolated hSpl were obtained from 3 different human spleens for each experiment (6 spleens) and cultured in triplicate alone or added to donor cultures at a 50:1 ratio or 500,000 hSpl/cm2 on Day 0. For Experiment 1, hSpl were added to one of three co-culture immune challenges at Day 0; allogeneic hSD-MSC, allogeneic hSDMSC-GFP, or lipopolysaccharide stimulation (LPS 10 μg/mL, Sigma-Aldrich, St. Louis, MO). For Experiment 2, hSpl were assigned to one of three co-culture immune challenges at Day 0; xenogeneic eqMSC, xenogeneic eqDFb, or lipopolysaccharide stimulation (LPS 10μg/mL, Sigma-Aldrich, St. Louis, MO). On Day –1, hSD-MSC wells were assigned to receive adenoviral vector transduction. Cells were transduced with AdGFP at 100 MOI for 2 h in Gey’s Balanced Salt Solution. Effective transduction methods with AdGFP have been validated in previously published work [19]. On Day 0, prior to the addition of the allogeneic hSpl cells, GFP expression was confirmed by fluorescent microscopy and recorded. In addition to co-cultures, hSpl were cultured alone. At day 0, hSpl were removed for cellular viability staining and flow cytometry analysis approximately two hours after co-cultures were established to serve as a baseline immune status. At Days 0 and 6, hSpl were harvested for cell surface marker content as measured by flow cytometry. 2.5. Flow Cytometry, Cell Numbers, and Cell Viability Flow cytometry (C-Flow Plus, Accuri, Ann Arbor, MI) was performed on hSpl subjected to extracellular staining to determine the proportion of cells positively-stained for binding of CD3, CD4, or CD8 antibodies. Human monoclonal antibodies for each immune cell subtype (FITCconjugated-anti-CD3, APC-conjugated anti-CD4, and PEconjugated anti-CD8, R&D Systems, Minneapolis, MN) were used to assess the potential formation of immune cell types. At Days 0 and 6, we investigated formation of CD3+ , CD4+ , and CD8+ cells; an unstained control and an antibody control for each respective antibody were used on each of flow cytometry analysis. Cell number and viability for both hSD-MSC and hSD-MSC/GFP were determined on Day 6. Cells were trypsinized, washed, and subjected to a trypan blue exclusion stain. Cells were counted in a hemacyometer; the number of live cells was recorded. 2.6. Statistical Analysis For co-culture experiments, cells isolated from one human joint (hSD-MSC or hSD-MSC/GFP or one animal (eqMSC or eqDFb) and an individual spleen (hSpl) were Copyright © 2012 SciRes. OJCB 4 S. S. JUMP ET AL. considered an n of 1. Data for quantitative outcomes of hSpl CD3+ , CD4+ , CD8+ number and proportion, as well as donor cell number and viability were presented as means ± standard error of the mean (SEM). Two factor analysis of variance (ANOVA) for Day (0 and 6) and hSpl condition (alone, hSD-MSC, hSD-MSC-GFP; or alone, eqMSC, eqDFb, LPS) was performed for hSpl number, donor cell number, and hSpl or donor cell viability. All analyses were performed using a comercially available statistical software package (Statistical Analysis Software, SAS 9.1). Statistical significance was accepted at p ><
0.05 3. Results Human spleens were obtained on ice, and splenoctyes were
harvested with initial high hSpl number and viability (>95%) (Figure 1(A))
and co-cultured with donor cells at Day 0 (Figure 1(B)). The proportion of gated
viable hSpl on the Day 0 of culture measurement by flow cytometry was in the
range of 65% - 74% (Figures 2(a) and 2(b)). The proportion of CD+ hSpl (CD3+ ,
CD8+ , plus CD4+ ) was <15% of total gated cells on Day 0 culture measurement by flow cytometry (Figures 3 and 4). Culture of hSpl alone resulted in a significant decrease in hSpl numbers and viability to an average of approximately 31% by Day 6 as measured by flow cytometry (Figures 2(a) and 2(b)). Co-culture of hSpl with allogeneic or xenogeneic donor cells did not further decrease hSpl numbers (Figures 2(a) and 2(b)). Both allogeneic and xenogeneic donor cells proliferated in cell co-culture with hSpl (Figure 5) and were of normal morphology with high efficiency of GFP expression demonstrated in hSD-MSC-GFP cells for the entire 6 days. (Figure 1(B), insert) Synovial-derived mesenchymal stromal cells were successfully transduced (hSD-MSC-GFP) 24h after transduction with AdGFP at 100 multiplicities of infection (100 MOI). (Day 0). In the allogeneic co-culture Experiment 1, the proportion of CD3+ and CD8+ hSpl was significantly greater on Day 6 (4-fold and 5-fold, respectively) regardless of culture condition as compared to Day 0. (Figures 3(a) and 3 (b)) On Day 6, hSpl cultured in LPS had a greater proportion of CD3+ hSpl than hSpl cultured alone. There was no change in the proportion of CD4+ hSpl on Day 6 versus 0 (Figure 3(c)). In the xenogeneic co-culture Experiment 2, the proportion of CD3+ and CD8+ hSpl was significantly greater on Day 6 (3-fold and 6-fold, respectively) regardless of culture condition as compared to Day 6 as in the allogeneic co-culture experiment and compared to Day 0. (Figures 4(a) and 4(b)) On Day 6, hSpl co-cultured with eqDFb had a lesser proportion of CD8+ hSpl than hSpl cultured alone. There was no change in the proportion of CD4+ Figure 1. (A) Photomicrograph (100× magnification) of a hemacytometer containing a cell dilution of human splenocytes stained with trypan blue stain after being digested from a human spleen. Viable hSpl are yellow and circular, and non-viable human splenocytes (hSpl) are blue and pyknotic. Each spleen sample that was digested had greater than 95% living cells prior to the start of the co-culture experiments (Day 0); (B) Photomicrograph (100× magnfication) of a co-culture containing hSpl (small circular cells) and human synovial-derived mesenchymal stromal cells (hSD-MSC) 100x magnification. Human SD-MSC transduced with AdGFP at 100 infectious Ad particles per cell, had >95%
expression of GFP under fluorescent microscopy at 24 hours (insert; 200×
magnification). cells between days (Figure 4(c)). 4. Discussion This study
provided evidence that, in a short-term culture environment, hSpl undergo a
natural selection and adaptation to cell culture conditions. In the allogeneic
or xenogenic co-culture conditions, no particular evaluated cell source
(allogeneic synovial or xenogeneic bonemarrow, or skin) or donor source (human
allogeneic or equine xenogeneic) promoted survival, inhibited death, or
promoted death of the lymphocyte cell fraction of hSpl. In addition, these data
showed that neither allogeneic nor Copyright © 2012 SciRes. OJCB S. S. JUMP ET
AL. 5 (a) (b) Figure 2. Number of gated (viable) hSpl on Day 0 and Day 6 in the
allogeneic Experiment 1 (Panel (a)) and the xenogeneic Experiment 2 (Panel
(b)). Abbreviations: hSpl = human splenocytes, hSpl + hSD – MSC = human
splenoctyes cocultured with human synovial-derived mesenchymal stromal cells,
hSpl + hSD – MSC/GFP = human splenocytes cocultured with human synovial-derived
mesenchymal stromal cells transduced with AdGFP, hSpl + eqMSC = human
splenoctyes co-cultured with equine mesenchymal stromal cells, hSpl + eqDFb =
human splenocytes co-cultured equine dermal fibroblasts, hSpl + LPS = human
splenocytes cocultured g/mL). Data aremwith
lipopolysaccharide (LPS, 10 SEM. The bracket (p±mean < 0.05) denotes a
statistical difference at Day 6 compared to Day 0. xenogeneic cells elicited a
lymphocytic/dendritic cell selection and no donor source promoted
proliferation, at least in vitro and for this short duration of primary
exposure. The short survival of immune cells in culture (Spl or peripheral
blood monocytes) limit in vitro studies. However, within these limitations, the
evidence suggested that allogeneic or xenogeneic cells may be immunotolerated,
at least initially, in a host environment. Our study was a first to investigate
the immunoresponse of a mixed population of hSpl to different tissue sources of
both allogeneic and xenogeneic origin cells and assessed three differing
mechanisms of immune challenge, including 1) cellular antigens, 2) adenoviral
antigens, and 3) lipopolysaccharide (LPS), a mediator of the inflammatory
immune reaction. We were unable to document (a) (b) (c) Figure 3. Human Human
splenocytes (hSpl) co-cultured with allogeneic synovial-derived mesenchymal
stromal cells (hSDMSC) and subjected to extracelluar staining using antibodies
specific for CD3, CD4, or CD8. The proportion of positive cells for each
antibody was determined by flow cytometry. (a) CD3+ hSpl on Day 0 and Day 6.
(b) CD8+ hSpl on Day 0 and Day 6. (c) CD4+ hSpl on Day 0 and Day 6.
Abbreviations: hSpl = human splenocytes, hSpl + hSD – MSC = human splenoctyes
co-cultured with human synovial-derived mesenchymal stromal cells, hSpl + hSD –
MSC/ GFP = human splenocytes co-cultured with human synovial-derived
mesenchymal stromal cells transduced with AdGFP, hSpl + LPS = human splenocytes
co-cultured with SEM.±g/mL). Data are mean mlipopolysaccharide
(LPS, 10 The bracket (p < 0.05)
denotes a statistical difference at Day 6 compared to Day 0. # denotes an
unexplained difference compared to other groups at Day 0. * denotes that hSpl +
LPS at Day 6 is significantly greater than Day 0. significant activation of CD+
immune cells or death of immune cells or death of allogeneic or xenogeneic
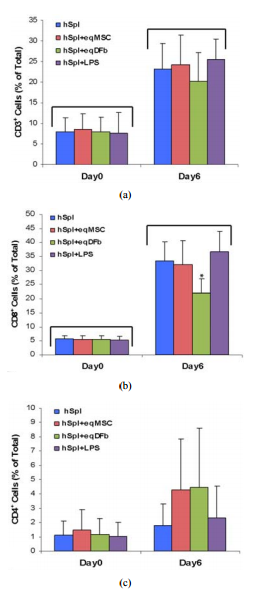
donor Copyright © 2012 SciRes. OJCB 6 S. S. JUMP ET AL. (a) (b) (c) Figure 4.
Human splenocytes (hSpl) co-cultured with xenogeneic equine bone-marrow derived
mesenchymal stromal cells (eqMSC) or dermal fibroblasts (eqDFb) and subjected
to extracelluar staining using antibodies specific for CD3, CD4, or CD8. The
proportion of positive cells for each antibody was determined by flow
cytometry. (a) CD3+ hSpl on Day 0 and Day 6. (b) CD8+ hSpl on Day 0 and Day 6.
(c) CD4+ hSpl on Day 0 and Day 6. Abbreviations: hSpl = human splenocytes, hSpl
+ eqMSC = human splenoctyes cocultured with equine mesenchymal stromal cells,
hSpl + eqDFb = human splenocytes co-cultured equine dermal fibroblasts, hSpl +
LPS = human splenocytes co-cultured ±g/mL).
Data are mean mwith lipopolysaccharide (LPS, 10 SEM. The bracket (p < 0.05) denotes a
statistical difference at Day 6 compared to Day 0. *denotes that hSpl + EqDFb
was significantly lower than other groups at Day 6. cells. Dermal fibroblasts
have not been stated to be immunoprivileged as has been claimed for the
synovial fibroblast or bone-marrow derived mesenchymal proFigure 5. Cell number
of human synovial-derived mesenchymal stromal cells (hSD-MSC) and human
synovial-derived mesenchymal stromal cells transduced with adenovirus
containing green fluorescent protein (hSD – MSC/GFP) on Day 0 and Day 6 as
determined by hemacytometer live cell count following a trypan blue exclusion
staining Data SEM. The bracket (p±are mean < 0.05) denotes a
statistical difference at Day 6 compared to Day 0. genitor and stromal cells,
but our data suggested that xenogeneic dermal fibroblasts may be
immunosuppressive to CD8+ cells and would warrant further investigation.
Importantly, further experiments in vivo would be necessary to more critically
assess immunotolerance, to include longer exposure to antigens, and evaluate a
second exposure to elucidate the cellular contributions and anamnestic response
to isolated allogeneic or xenogeneic cells. To our knowledge, our splenocyte
co-culture system is novel and contributed initial information on tolerance of
these mixed immune cells to donor cells on short term exposure and supports
previous publications claiming allogeneic juvenile chondroctyes exhibit
immunotolerance [27]. Previous work has also provided evidence that synovial
cells have immune privilege and will not be rejected by a host organism
[28,29]. Some studies supported the tolerance of allo- or xenotransplants for
tissue healing [30,31]. An additional study showed that xenogeneic chondrocytes
can successfully repair full-thickness cartilage defects [32]. However, in a
recent 2010 study, xenotransplantation of porcine chondrocytes into rabbits was
unsuccessful for long term cartilage healing [11]. Our study provided a unique
contribution to the relatively limited body of work in the literature using
this type of co-culture model. Our data is in agreement with previous
literature suggesting that mesenchymal stromal cells maybe immunotolerated or
immunoprivileged [15, 17,33]. Findings in our human allogeneic experiment
demonstrated that no detectable immune activation was prompted; however, an
immunosuppression was not observed as in other studies. In cell culture, MSCs
have also been able to suppress T cell proliferation [13], sugCopyright © 2012
SciRes. OJCB S. S. JUMP ET AL. 7 gesting MSC, including stem cells, are not
only immunotolerated, but may be immunosuppressive [15]. Maccario and
colleagues observed a decrease in the formation of CD8+ T cells and natural
killer lymphocytes in favor of an expansion of CD4+ lymphocytes in response to
allogeneic MSC [17]. However, in our xenogeneic experiments, the evidence
suggested that equine dermal fibroblasts may play a role in the inhibition of
an immune response. Our data showed less of an increase in CD8+ cells after
exposure to xenogeneic dermal fibroblasts. Additional evidence has shown that
fibroblasts, both autologous or allogeneic cells, resulted in no increase in
T-cell proliferation; however, this effect required a modification of CD80
expression on the surface of the cell [12]. Data is accumulating that suggests
an immunotolerance to selected allogeneic and xenogeneic isolated cells. Our
data showed variability in human splenocyte initial cell proportions from human
to human, demonstrated as wide standard deviations in isolated hSpl numbers and
percentages of CD+ cells. The variability of splenocyte populations under our
conditions are likely explained by differences in age, gender, and
hematological parameters of our human spleen donors. Human spleens were
acquired from an organ donor center, without exclusion for age, gender, or
cause of death. Previous health history of the human spleen donors may have
altered the proportion of various cell types in the spleen including mature lymphocytes,
T-helper cells, dendritic cells, or natural killer/ cytotoxic T cells. The
heterogeneous human population providing hSpl sources probably added
variability to our co-culture condition responses, but represent a typical
recipient population of humans. Despite the variability inherent in using hSpl
from multiple human donors, we were able to demonstrate the natural selection
and adaptation of hSpl to the cell culture environment in a shortterm cell
culture study, minimal lymphocyte activation by allogeneic and xenogeneic cell
exposure and the ability of donor allogeneic and xenogeneic cells to thrive in
direct contact with a mixed population of immune cells. The clinical
applicability of our work extends to potential cell-based strategies for the repair
of human tissue. Allogeneic or xenogeneic cell sources could serve as a
powerful clinical tool to enhance current medical practices that promote
healing. Finding a potential cell- or tissue-based treatment for cartilage is
dependent upon further understanding of the immunoresponse of cells, including
synovial lining cells or chondrocytes. Further characterizing the
immunoresponse may enable potential molecular or other cellular modifications
to enable specific cells to “tolerate” an immune response or modify the native
immune response. It is possible that specific cells could be engineered to
modify the acute immune response in vivo, ahead of or in conjunction cellular
injection or transplantation 5. Conclusions We uniquely investigated the
immunoresponse of a mixed population of hSpl to three different mechanisms of
allogeneic or xenogeneic challenge: 1) cellular antigens 2) cellular/adenoviral
antigens and 3) lipopolysaccharide (LPS). In our in vitro model, neither
allogeneic nor xenogeneic cells elicited a lymphocyte/dendritic cell
immunoresponse. Our study provided evidence that, in a shortterm culture
environment, hSpl undergo a natural selection and adaptation to cell culture
conditions. In hSpl cocultures with hSD-MSC, hSD-MSC-GFP, eqDFb, or eqMSC, CD3+
and CD8+ hSpl increased in proportion and decreased in number with time in
culture, but not with exposure to allogeneic or xenogeneic cell antigens. The
evidence suggested that allogeneic or xenogeneic may be immunotolerated in the
short term and upon first exposure by human recipients. Further studies to
characterize the long-term response in vivo and the anemnestic response is
warranted 6. Acknowledgements Dr. Jump was supported by a Fellowship at The
Ohio State University from the Sports Medicine Center. Dr. Bertone was
supported by NIH/NIAMS grant number K08AR4920101. The work was supported by the
Trueman Endowment. We would like to acknowledge and thank Dr. Akikazu Ishihara
for his assistance in the statistical and data analysis and for proofreading
and editing our mansucript. Additionally, we offer a special thanks to Dr.
Prosper Boyaka and his laboratory personnel, Dr. Junbae Jee and Haley Steiner,
for assistance with technical guidance and methodological expertise in
performing flow cytometry.