
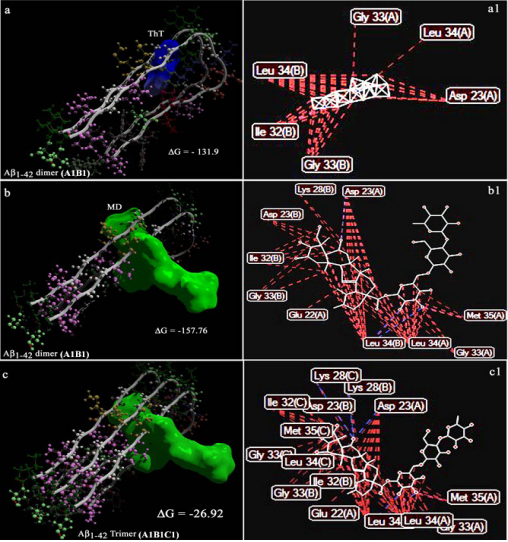
 Confirmation of the Experimentally-Proven Therapeutic Utility of Madecassoside in an Aβ1-42 Infusion Rat Model of Alzheimer’s Disease by in Silico Analyses Abdullah Al Mamun1, Michio Hashimoto1*, Shahdat Hossain1,2, Masanori Katakura1, Hiroyuki Arai3, Osamu Shido1 1 Department of Environmental Physiology, Shimane University Faculty of Medicine, Izumoshi, Japan 2 Laboratory of Alternative Medicine and Behavioral Neurosciences, Department of Biochemistry and Molecular Biology, Jahangirnagar University, Dhaka, Bangladesh 3 Department of Geriatrics and Gerontology, Division of Brain Sciences, Institute of Development, Aging and Cancer, Tohoku University, Sendai, Japan Email: mamun104@gmail.com, * michio1@med.shimane-u.ac.jp, shahdat@dhaka.net, katakura@med.shimane-u.ac.jp, harai@idac.tohoku.ac.jp, o-shido@med.shimane-u.ac.jp Received 9 April 2015; accepted 6 June 2015; published 9 June 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract The accumulation of amyloid β peptide 1 - 42 (Aβ1-42) in the brain of Alzheimer’s disease (AD) patients is known to be associated with neurodegeneration and memory impairment. More recently, we reported that madecassoside, an active component of Centella asiatica, improved memory impairment in an Aβ1-42 infusion rat model of AD, ameliorated neurotoxicity in SH-SY5Y cells, and inhibited in vitro Aβ1-42 fibril formation. In the present study, we investigated the utility of in silico analyses in corroborating observed in vivo and in vitro effects of madecassoside in AD to further assess the therapeutic benefits of madecassoside. The 3D structure of Aβ1-42 was downloaded from the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank (PDB). The binding of madecassoside to Aβ1-42 was assessed by molecular docking. The chemical structure of madecassoside was modeled and converted to the PDB format. Madecassoside was found to successfully dock with Aβ1-42. Computational demonstration of the binding of madecassoside to Aβ1-42 further corroborated the inhibitory effect of madecassoside on Aβ1-42 fibrillogenesis which was demonstrated in our previous study. These data showed the potential utility of madecassoside as a preventive medication in Aβ1-42-induced neurodegenerative diseases such as AD. * Corresponding author. A. A. Mamun et al. 38 Keywords Alzheimer’s Disease, Centella asiatica, Madecassoside, Ayurveda, in Silico, Drug Designing 1. Introduction Alzheimer’s disease (AD) is a dementia-related neurodegenerative disease. AD is pathologically characterized by fibrillar deposition of amyloid β (Aβ) peptides in the brain [1]. The Aβ1-42 peptide is the predominant constituent of deposits observed in the affected brain of AD patients [2]. The prevalence of AD is 10% in 65-year-old people, and it increases to 50% in individuals aged over 80 years old, indicating an increasing prevalence of AD in elderly people. Alzheimer’s disease International (ADI) estimates that there are currently 30 million people with dementia worldwide, with 4.6 million new cases annually (one new case every 7 s). The number of people affected is estimated to reach more than 100 million by 2050 [3]. AD is the fourth leading cause of death in individuals over the age of 65. AD is a progressive neurodegenerative disorder with insidious onset characterized by severe decline in episodic memory. Madecassoside, a highly polyphenolic compound, is the major triterpene glycoside found in Centella asiatica [4]. We previously reported that purified madecassoside inhibits in vitro Aβ1-42 fibril formation [5]. More recently, we reported that long-term oral administration of purified madecassoside protected against spatial memory impairment in Aβ1-42-infused AD model rats and inhibited Aβ1-42 fibril formation, as indicated by ThioflavinT (ThT) fluorometry, laser scanning microscopy, and transmission electron microscopy [6]. We further demonstrated that co-treatment with madecassoside significantly attenuated Aβ1-42-induced apoptosis in SHSY5Y cells, with concurrent decreases in the levels of lipid peroxidation, Aβ1-42 burden, and TNF-α and increases in hippocampal levels of Brain derived neurotrophic factor (BDNF) and postsynaptic density protein (PSD-95) [6]. Our results clearly indicated that madecassoside provided therapeutic benefits in AD. Despite a lack of clarification regarding the interaction between madecassoside and Aβ1-42, agents capable of targeting amyloid toxicity leading to memory impairment in AD have been extensively researched. Although we previously demonstrate the therapeutic utility of madecassoside in animal models, the exact mechanismofaction of madecassoside is yet to be determined. Therefore in this study, we developed models of the binding of madecassoside, as the ligand, to Aβ1-42, as the receptor, to further evaluate the anti-Aβ1-42 fibrillation effect of madecassoside demonstrated in our previous study [6]. Structure (target)-based drug design by molecular docking represents an interesting venue to identify and optimize drug candidates by examining and modeling molecular interactions between ligands and target macromolecules. The fluorescent dye Thioflavin-T (ThT) has become among the most widely used “gold standards” for selectively binding, staining and identifying amyloid fibrils both in vivo and in vitro. The large enhancement of its fluorescence emission upon binding to amyloid fibers makes ThT a particularly powerful and convenient tool. For the extraordinary ability of ThT to recognize and bind with the amyloid fibrils, here, we used the ThT-fibril interactions as positive control. We docked madecassoside as ligand to the Aβ1-42, as receptors. We analyzed the binding of madecassoside in the context of the interaction of ThT with Aβ1-42. 2. Methods 2.1. In Silico Studies The software/web servers used were Marvin Sketch, Molegro Virtual Docker, Patch Dock, and Rossetta Dock. 2.2. Preparation of Docking Materials: ThT and Madecassoside (Ligands) and Aβ1-42 Dimers and Trimers (Receptors) The canonical Simplified Molecular Input Line Entry System (SMILES) strings of ThT (Chemical Identification number: 16,953; Figure 1) and madecassoside (Chemical Identification number: 45,356,919; Figure 1) were submitted to Marvin 5.7.0 [7] to generate the three-dimensional (3D) structure of each ligand molecule. The 3D structures of ThT and madecassoside were subsequently energy-minimized and converted to the Protein Data Bank (PDB) file format using the Molegro Virtual Docker (MVD) [8]. Aβ1-42 (PDB ID: 2BEG) was downloaded A. A. Mamun et al. 39 Figure 1. The ligand molecules: thioflavin T (ThT) and madecassoside (MD). from the PDB as a receptor for ThT and madecassoside docking. 2BEG is a 3D nuclear magnetic resonance solution structure of Aβ1-42, comprising residues 18 - 42 of a β-strand-turn-β-strand motif, and contains two intermolecular β-sheets that are formed by residues 18 - 26 (β 1) and 31 - 42 (β 2). 2BEG is a homopentamer, namely composed of A, B, C, D, and E monomer of Aβ1-42. Each monomer of 2BEG comprises 10 coordinate models [9]. The coordinates of model 1 of A monomer (A1), model 1 of B monomer (B1), and model 1 of C monomer (C1) were split from the composite 2BEG PDB file by MVD. Subsequently, the dimer, A1B1, was generated by Rossetta Dock [10] based on energy minimization. A trimer, A1B1C1, was also generated for madecassoside docking. 2.3. Molecular Docking of ThT and Madecassoside onto Aβ1-42 Dimer and Trimer 2.3.1. Docking with MVD The docking simulation was performed using the docking software MVD [8]. MVD is an automated docking software program with fast processing that automatically adds the missing hydrogen atoms of the ligand and receptor molecules, if any. The software also has a module to create a surface over the receptor molecule and identify potential binding sites for its activity. The program gives 10 conformational positions or so-called poses for the ligand and returns the five best poses with Mole Dock Score (equivalent to energy of binding/docking energy) and other thermodynamically calculated values. MVD also presents hydrogen bond information together with other thermodynamic values that suggest the formation of stable complexes between ligands and receptor molecules. MVD performs flexible ligand docking with optimization of the ligand geometry during docking. 2.3.2. Docking with Patch Dock Patch Dock [11] is a geometry-based molecular docking algorithm designed to find docking transformations that yield good molecular shape complementarity. Such transformations, when applied, induce both wide interface areas and small amounts of steric clashes. A wide interface ensures the inclusion of several matched local features of docked molecules with complementary characteristics. 3. Results 3.1. Molegro Virtual Docker (MVD) Docking performed by MVD provided the five best poses with corresponding Mol Dock score values and other thermodynamically calculated values. The 3D structures of bests coring dockings are shown in Figures 2(a)-(c). Steric interactions of both ThT and madecassoside, as ligands, with Aβ1-42 (the receptor) were also evaluated A. A. Mamun et al. 40 Figure 2. The best docking poses of the ligands, thioflavin T (ThT) and madecasoside (MD) with the receptors (Aβ1-42). ThT in pose 1 docked onto the A1B1 dimer (a), MD in pose 1 docked on toA1B1 dimer (b) and A1B1C1 trimer (c). Each monomeric strand, consisting of in-register parallel β1- and β2-sheets, runs perpendicularly along the long axis of the fiber. Interaction (binding) energies (−ΔG), shown as MolDock scores, are shown in (a)-(c). (a1)-(c1): contact (binding) maps of the interactions between atoms of ThT/MD and amino acid residues of the dimer/trimer (see also Table 1). Contact maps were visualized by Molegro Virtual Docker (MVD). Blue lines, formation of hydrogen bonds; red lines, steric interactions. A1, B1, and C1 are the corresponding coordinates of model 1 for the A, B, and C monomers of Aβ1-42, respectively. with MVD. Maps of the interactions of ThT with amino acid residues of the Aβ1-42 dimer (A1B1) are shown in Figure 2(a1). The docking of madecassoside to trimeric Aβ1-42 (A1B1C1) and its interaction map are shown in Figure 2(c1). The binding of madecassoside to the dimer was stronger than that of the trimer, as demonstrated by decreased binding energies (Figures 2(b)-(c)). Amino acids involved in the docking of madecassoside to the dimer (A1B1) and trimer (A1B1C1) were visualized by contact maps of the interactions between atoms of madecassoside and the amyloid amino acid residues using MVD (Figures 2(b1)-(c1), and Table 1). The amino acid residues of the A1B1 dimer found to sterically interact with ThT were Asp23, Gly33, and Leu34 of the A1 monomer and Ile32, Gly33, and Leu34 of the B1 monomer (Figures 2(a)-(a1)). Madecassoside sterically interacted with Glu22, Asp23, Gly33, Leu34 and Met35 of the A1 monomer, and Asp23, Lys28, Ile32, Gly33, Leu34 of the B1 monomer. These results clearly indicate that ThT and madecassoside have common binding sites at A. A. Mamun et al. 41 Table 1. Interaction sites of thioflavin T (ThT) and madecassoside (MD) with the amino acid residues of amyloid dimer (A1B1) and trimmer (A1B1C1). Amino acid residues Ala Glu Asp Val Gly Ser Asn Lys Gly Ala Ile Ile Gly Leu Met Val Gly Gly Val Val Ile Ala 1 - 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 ThT Dimer A1 B1 MD Dimer A1 B1 Trimer A1 B1 C1 Asp23 of the β1-sheet and Gly33 and Leu34 of the β2-sheet of the A1 monomer. These two ligands also had identical binding sites for the B1 monomer. In addition, the higher free energy of binding of madecassoside than ThT indicates stronger binding of madecassoside to the dimer (A1B1). Madecassoside was also found to bind with the amyloid trimer (A1B1C1); however, the binding energy was lower than that observed for the binding of madecassoside to the dimer (A1B1). 3.2. Patch Docker After performing molecular docking with MVD, we performed molecular docking of ThT and madecassoside using Patch Dock to confirm the ability of madecassoside to bind amyloid A1B1 dimer and A1B1C1 trimer (Figure 3). The algorithm used in Patch Dock performs rigid docking, with surface variability/flexibility implicitly addressed through liberal intermolecular penetration. Geometric shape complementarity scores were higher for madecassoside than for ThT when docked with both the dimer and the trimer. Approximate complex interface areas (receptor-ligand) and atomic contact energies were also higher for madecassoside (Table 2), suggesting stronger binding of madecassoside to the A1B1 dimer and A1B1C1 trimer than that of ThT. 4. Discussion Aβ1-42 peptides are important drug development targets because of their crucial role in neuronal diseases, including AD. Thus, the design of drugs that disrupt the formation of Aβ1-42-Aβ1-42 interfaces is particularly important for the development of therapies for the inhibition of Aβ1-42 fiber formation and/or the dismantling of preformed Aβ1-42 fibers. In other words, the development of Aβ1-42 fibrillation antagonists may provide an increased understanding of the pathogenesis of AD, leading to the development of more effective therapies. We previously demonstrated that co-incubation of free Aβ1-42 monomers with madecassoside prevented fiber polymerization [5]. The inhibitory effect of madecassoside on Aβ1-42 fibril formation corroborated previous ThT fluorescence spectroscopy and transmission electron microscopy findings [6]. In this study, our intention was to computationally model the interaction of madecassoside with Aβ1-42 by molecular docking. Ligand binding sites for Aβ1-42 were examined using high-resolution 3D coordinates obtained from the PDB. Finally, we sought to obtain information regarding the mechanism of madecassoside-induced inhibition of amyloid fibril formation by computationally modeling the binding of ThT with Aβ1-42. We previously reported that Aβ fibers bind to ThT [12]-[15]. ThT is one of the most widely used amyloid dyes used in the hundreds of amyloid studies published every year. The intensive use of ThT as an in vitro marker of amyloid formation has provided substantial information regarding the mechanism of ThT binding. Further studies aim to fully determine common structure(s) of the amyloid peptide recognized by ThT. Within the past several years, a series of critical experimental analyses of ThT have elucidated, in detail, many of the atomiclevel interactions underlying ThT binding and fluorescence. Because several recent reports have discussed the broad range of amyloid staining dyes [16]-[18], in the present study, we instead focused on the atomiclevel mechanisms underlying the binding of ThT to amyloid fibrils. Amyloid fibrils may have a specific ThT binding site that sterically “locks” the bound dye, leading to the enhancement of ThT fluorescence. These properties of ThT made it an ideal positive reference compound for this A. A. Mamun et al. 42 Figure 3. The best scoring results of the Patch Docking of Thioflavin T (ThT) (a)-(b) and madecassoside (MD) (c)-(d) with theA1B1 dimer and A1B1C1 trimer. Ligands (ThT and MD) are represented by green surface-fill representation and the receptors (A1B1 dimer and A1B1C1 trimer) are in ribbons + surface presentation. Table 2. Patch docking of thioflavin T (ThT) and madecassoside (MD) with the amyloid dimer (A1B1) and trimer (A1B1C1). Docking (Receptor vs. Ligands) Score Area ACE A1B1 dimer vs. ThT 2942 356.20 −314.68 A1B1 dimer vs. MD 6130 789.50 −470.03 A1B1C1 trimer vs. ThT 2820 307.50 −261.45 A1B1C1 trimer vs. MD 6586 845.50 −460.37 Score: Geometric shape complementarity score; Area: Approximate interface area of the complex (receptor-ligand); ACE: Atomic contact energy; A1B1 dimer: Generated by feeding the A1 and B1 model 1 of 2BEG to Rosetta Server; A1B1C1 trimer: Generated feeding the A1, B1 and C1 coordinate model 1 of 2BEG; ThT:Thioflavin T and MD: madecassoside. study. Structure-based drug design has made substantial contributions to drug discovery, including the development of treatments for cancer and drug-resistant infectious diseases. Computational structure-based drug design may, therefore, facilitate the development of novel treatments for AD. We recently reported that asiaticoside, another polyphenol compound of Centella asiatica, also inhibited fibril formation [19]. Therefore, the docking of madecassoside with the Aβ1-42 dimer/trimer was used to corroborate our previous findings regarding the in vivo and in vitro inhibitory effects of madecassoside on fibril formation and memory impairment in an AD rat model [6]. Aggregation-prone amino acids, i.e., hotspots for Aβ1-42 aggregation, are located at residues 17 - 22 and 32 - 42 [19]. While docked with the dimer/trimer, madecassoside displayed greater binding affinity, according to binding energy, than ThT. These results demonstrate that madecassoside is capable of binding amyloid fibers, potentially providing an explanation for the reduced amyloid burden observed in the brains of madecassoside-administered AD model rats and concurrent inhibition of in vitro fibril formation [6]. Notably, residues 17 - 21 and residues 31 - 42 are β-sheet forming amino acids engaged in monomer-monomer (dimer) inter surface interactions [19]. The common features between MVD and Patch Docke were displayed in their common amino acid binding sites for madecassoside: Asp23 and Leu34 of the Aβ1-42 (analysis not shown). These results again suggest that inter surface interaction sites is the most promising target sites for ligands that potentially inhibit amyloid fibril interactions, such as madecassoside. Hossain et al. (2009) previously reported that the dimeric/trimeric form of Aβ1-42 acts as one of the seeding units for Aβ1-42 fibril formation [12]. Therefore, we assessed the molecular docking of the dimeric/trimeric structures of Aβ1-42 with madecassoside. Polyphenols are capable of forming hydrogen bonds with amyloids and other proteins [20] [21]. In the present in silico study, madecassoside was A. A. Mamun et al. 43 found to affect Aβ1-42 fibril formation through binding to amino acid residues 17 - 21 and 31 - 42, the most aggregation-sensitive regions of Aβ1-42, and forming hydrogen bonds with Aβ1-42. The neurotoxicity of Aβ1-42 involves the in vivo conformational transition from soluble α-helical to insoluble β-sheet forms of the peptide following release from the cell membrane into the surrounding aqueous environment. 5. Conclusion Herein, we demonstrate the successful molecular docking of madecassoside on to Aβ1-42, with a higher affinity of binding than that with the thioflavin T, consistent with the inhibitory effects of madecassoside on in vitro fibril formation and memory impairment in a rat model of AD. Our in silico results further provide compelling evidence for the utility of triterpene glycosides as a preventive medication for neurodegenerative diseases such as Aβ1-42 aggregation-induced AD. Acknowledgements This work was supported in part by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education Culture, Sports, Science and Technology, Japan. The authors would like to thank Enago (www.enago.jp) for the English language review. References [1] Selkoe, D.J. (1991) The Molecular Pathology of Alzheimer’s Disease. Neuron, 6, 487-498. http://dx.doi.org/10.1016/0896-6273(91)90052-2 [2] Iwatsubo, T., Odaka, A., Suzuki, N., Mizusawa, H., Nukina, N. and Ihara, Y. (1994) Visualization of Aβ 42(43) and Aβ40 in Senile Plaques with End-Specific Aβ Monoclonals: Evidence That an Initially Deposited Species Is Aβ 42(43). Neuron, 13, 45-53. http://dx.doi.org/10.1016/0896-6273(94)90458-8 [3] Ferri, C.P., Prince, M., Brayne, C., Brodaty, H., Fratiglioni, L., Ganguli, M., Hall, K., Hasegawa, K., Hendrie, H., Huang, Y.Q., Jorm, A., Mathers, C., Menezes, P.R., Rimmer, E. and Scazufca, M. (2005) Global Prevalence of Dementia: A Delphi Consensus Study. The Lancet, 366, 2112-2117. http://dx.doi.org/10.1016/S0140-6736(05)67889-0 [4] Wanasuntronwong, A., Tantisira, M.H., Tantisira, B. and Watanabe, H. (2012) Anxiolytic Effects of Standardized Extract of Centella asiatica (ECa 233) after Chronic Immobilization Stress in Mice. Journal of Ethnopharmacology, 143, 579-585. http://dx.doi.org/10.1016/j.jep.2012.07.010 [5] Hossain, S., Hashimoto, M., Katakura, M. and Shido, O. (2013) Asiaticoside and Madecassoside, Two Major Glycosides of Centella asiatica Inhibit the in Vitro Amyloid Beta Peptide Aβ1-42 Fibrillation—Assessed by FCS, Capable of Detecting Single Molecular Movement and Interaction. Proceedings of the 11th International Conference on Alzheimer’s & Parkinson’s Diseases, Florence, 6-10 March 2013, 245. [6] Mamun, A.A., Hashimoto, M., Katakura, M., Matsuzaki, K., Hossain, S., Arai, H. and Shido, O. (2014) Neuroprotective Effect of Madecassoside Evaluated Using Amyloid β1-42-Mediated in Vitro and in Vivo Alzheimer’s Disease Models. International Journal of Indigenous Medicinal Plants, 47, 1669-1682. [7] Marvin (2011) Marvin Was Used for Drawing, Displaying and Characterizing Chemical Structures, Substructures and Reactions, Marvin 5.7. [8] Thomsen, R. and Christensen, M.H. (2006) MolDock: A New Technique for High-Accuracy Molecular Docking. Journal of Medicinal Chemistry, 49, 3315-3321. http://dx.doi.org/10.1021/jm051197e [9] Lührs, T., Ritter, C., Adrian, M., Riek-Loher, D., Bohrmann, B., Döbeli, H., Schubert, D. and Riek, R. (2005) 3D Structure of Alzheimer’s Amyloid-Beta (1-42) Fibrils. roceedings of the National Academy of Sciences of the United States of America, 102, 17342-17347. http://dx.doi.org/10.1073/pnas.0506723102 [10] Lyskov, S. and Gray, J.J. (2008) The RosettaDock Server for Local Protein-Protein Docking. Nucleic Acids Research, 36, W233-W238. http://dx.doi.org/10.1093/nar/gkn216 [11] Duhovny, D., Nussinov, R. and Wolfson, H.J. (2002) Efficient Unbound Docking of Rigid Molecules. In: Guigó, R. and Gusfield, D., Eds., Proceedings of the 2nd Workshop on Algorithms in Bioinformatics (WABI) Rome, Italy, Lecture Notes in Computer Science (LNCS) 2452, Springer Verlag, Berlin Heidelberg, 185-200. [12] Hossain, S., Hashimoto, M., Katakura, M., Miwa, K., Shimada, T. and Shido, O. (2009) Mechanism of Docosahexaenoic Acid-Induced Inhibition of in Vitro Aβ1−42 Fibrillation and Aβ1−42-Induced Toxicity in SH-S5Y5 Cells. Journal of Neurochemistry, 111, 568-579. http://dx.doi.org/10.1111/j.1471-4159.2009.06336.x [13] Hashimoto, M., Hossain, S., Yamashita, S., Katakura, M., Tanabe, Y., Fujiwara, H., Gamoh, S., Miyazawa, T., Arai, H., Shimada, T. and Shido, O. (2008) Docosahexaenoic Acid Disrupts in Vitro Amyloid β1−40 Fibrillation and Conco- A. A. Mamun et al. 44 mitantly Inhibits Amyloid Levels in Cerebral Cortex of Alzheimer’s Disease Model Rats. Journal of Neurochemistry, 107, 1634-1646. http://dx.doi.org/10.1111/j.1471-4159.2008.05731.x [14] Hashimoto, M., Hossain, S., Katakura, M., Mamun, A.A. and Shido, O. (2015) The Binding of Aβ1−42 to Lipid Rafts of RBC Is Enhanced by Dietary Docosahexaenoic Acid in Rats: Implicates to Alzheimer’s Disease. Biochimica et Biophysica Acta, 1848, 1402-1409. http://dx.doi.org/10.1016/j.bbamem.2015.03.008 [15] Hashimoto, M., Hossain, S., Hossain, S., Rahman, A., Shimada, T. and Shido, O. (2011) Docosahexaenoic Acid Withstands the Aβ25-35-Induced Neurotoxicity in SH-SY5Y Cells. The Journal of Nutritional Biochemistry, 22, 22-29. http://dx.doi.org/10.1016/j.jnutbio.2009.11.005 [16] Groenning, M. (2009) Binding Mode of Thioflavin T and Other Molecular Probes in the Context of Amyloid FibrilsCurrent Status. Journal of Chemical Biology, 3, 1-18. http://dx.doi.org/10.1007/s12154-009-0027-5 [17] Kim, Y., Lee, J.H., Ryu, J. and Kim, D.J. (2009) Multivalent & Multifunctional Ligands to Beta-Amyloid. Current Pharmaceutical Design, 15, 637-658. http://dx.doi.org/10.2174/138161209787315648 [18] Nilsson, K.P. (2009) Small Organic Probes as Amyloid Specific Ligands-Past and Recent Molecular Scaffolds. FEBS Letters, 583, 2593-2599. http://dx.doi.org/10.1016/j.febslet.2009.04.016 [19] Hossain, S., Hashimoto, M., Katakura, M., Mamun, A.A. and Shido, O. (2015) Medicinal Value of Asiaticoside for Alzheimer’s Disease as Assessed Using Single-Molecule-Detection Fluorescence Correlation Spectroscopy, LaserScanning Microscopy, Transmission Electron Microscopy, and in Silico Docking. BMC Complementary and Alternative Medicine, 15, in Press. http://dx.doi.org/10.1186/s12906-015-0620-9 [20] Sirk, T.W., Friedman, M. and Brown, E.F. (2011) Molecular Binding of Black Tea Theaflavins to Biological Membranes: Relationship to Bioactivities. Journal of Agricultural and Food Chemistry, 59, 3780-3787. http://dx.doi.org/10.1021/jf2006547 [21] Stefani, M.S. (2013) Protein Folding and Aggregation into Amyloid: The Interference by Natural Phenolic Compounds. International Journal of Molecular Sciences, 14, 12411-12457. http://dx.doi.org/10.3390/ijms140612411
Confirmation of the Experimentally-Proven Therapeutic Utility of Madecassoside in an Aβ1-42 Infusion Rat Model of Alzheimer’s Disease by in Silico Analyses Abdullah Al Mamun1, Michio Hashimoto1*, Shahdat Hossain1,2, Masanori Katakura1, Hiroyuki Arai3, Osamu Shido1 1 Department of Environmental Physiology, Shimane University Faculty of Medicine, Izumoshi, Japan 2 Laboratory of Alternative Medicine and Behavioral Neurosciences, Department of Biochemistry and Molecular Biology, Jahangirnagar University, Dhaka, Bangladesh 3 Department of Geriatrics and Gerontology, Division of Brain Sciences, Institute of Development, Aging and Cancer, Tohoku University, Sendai, Japan Email: mamun104@gmail.com, * michio1@med.shimane-u.ac.jp, shahdat@dhaka.net, katakura@med.shimane-u.ac.jp, harai@idac.tohoku.ac.jp, o-shido@med.shimane-u.ac.jp Received 9 April 2015; accepted 6 June 2015; published 9 June 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract The accumulation of amyloid β peptide 1 - 42 (Aβ1-42) in the brain of Alzheimer’s disease (AD) patients is known to be associated with neurodegeneration and memory impairment. More recently, we reported that madecassoside, an active component of Centella asiatica, improved memory impairment in an Aβ1-42 infusion rat model of AD, ameliorated neurotoxicity in SH-SY5Y cells, and inhibited in vitro Aβ1-42 fibril formation. In the present study, we investigated the utility of in silico analyses in corroborating observed in vivo and in vitro effects of madecassoside in AD to further assess the therapeutic benefits of madecassoside. The 3D structure of Aβ1-42 was downloaded from the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank (PDB). The binding of madecassoside to Aβ1-42 was assessed by molecular docking. The chemical structure of madecassoside was modeled and converted to the PDB format. Madecassoside was found to successfully dock with Aβ1-42. Computational demonstration of the binding of madecassoside to Aβ1-42 further corroborated the inhibitory effect of madecassoside on Aβ1-42 fibrillogenesis which was demonstrated in our previous study. These data showed the potential utility of madecassoside as a preventive medication in Aβ1-42-induced neurodegenerative diseases such as AD. * Corresponding author. A. A. Mamun et al. 38 Keywords Alzheimer’s Disease, Centella asiatica, Madecassoside, Ayurveda, in Silico, Drug Designing 1. Introduction Alzheimer’s disease (AD) is a dementia-related neurodegenerative disease. AD is pathologically characterized by fibrillar deposition of amyloid β (Aβ) peptides in the brain [1]. The Aβ1-42 peptide is the predominant constituent of deposits observed in the affected brain of AD patients [2]. The prevalence of AD is 10% in 65-year-old people, and it increases to 50% in individuals aged over 80 years old, indicating an increasing prevalence of AD in elderly people. Alzheimer’s disease International (ADI) estimates that there are currently 30 million people with dementia worldwide, with 4.6 million new cases annually (one new case every 7 s). The number of people affected is estimated to reach more than 100 million by 2050 [3]. AD is the fourth leading cause of death in individuals over the age of 65. AD is a progressive neurodegenerative disorder with insidious onset characterized by severe decline in episodic memory. Madecassoside, a highly polyphenolic compound, is the major triterpene glycoside found in Centella asiatica [4]. We previously reported that purified madecassoside inhibits in vitro Aβ1-42 fibril formation [5]. More recently, we reported that long-term oral administration of purified madecassoside protected against spatial memory impairment in Aβ1-42-infused AD model rats and inhibited Aβ1-42 fibril formation, as indicated by ThioflavinT (ThT) fluorometry, laser scanning microscopy, and transmission electron microscopy [6]. We further demonstrated that co-treatment with madecassoside significantly attenuated Aβ1-42-induced apoptosis in SHSY5Y cells, with concurrent decreases in the levels of lipid peroxidation, Aβ1-42 burden, and TNF-α and increases in hippocampal levels of Brain derived neurotrophic factor (BDNF) and postsynaptic density protein (PSD-95) [6]. Our results clearly indicated that madecassoside provided therapeutic benefits in AD. Despite a lack of clarification regarding the interaction between madecassoside and Aβ1-42, agents capable of targeting amyloid toxicity leading to memory impairment in AD have been extensively researched. Although we previously demonstrate the therapeutic utility of madecassoside in animal models, the exact mechanismofaction of madecassoside is yet to be determined. Therefore in this study, we developed models of the binding of madecassoside, as the ligand, to Aβ1-42, as the receptor, to further evaluate the anti-Aβ1-42 fibrillation effect of madecassoside demonstrated in our previous study [6]. Structure (target)-based drug design by molecular docking represents an interesting venue to identify and optimize drug candidates by examining and modeling molecular interactions between ligands and target macromolecules. The fluorescent dye Thioflavin-T (ThT) has become among the most widely used “gold standards” for selectively binding, staining and identifying amyloid fibrils both in vivo and in vitro. The large enhancement of its fluorescence emission upon binding to amyloid fibers makes ThT a particularly powerful and convenient tool. For the extraordinary ability of ThT to recognize and bind with the amyloid fibrils, here, we used the ThT-fibril interactions as positive control. We docked madecassoside as ligand to the Aβ1-42, as receptors. We analyzed the binding of madecassoside in the context of the interaction of ThT with Aβ1-42. 2. Methods 2.1. In Silico Studies The software/web servers used were Marvin Sketch, Molegro Virtual Docker, Patch Dock, and Rossetta Dock. 2.2. Preparation of Docking Materials: ThT and Madecassoside (Ligands) and Aβ1-42 Dimers and Trimers (Receptors) The canonical Simplified Molecular Input Line Entry System (SMILES) strings of ThT (Chemical Identification number: 16,953; Figure 1) and madecassoside (Chemical Identification number: 45,356,919; Figure 1) were submitted to Marvin 5.7.0 [7] to generate the three-dimensional (3D) structure of each ligand molecule. The 3D structures of ThT and madecassoside were subsequently energy-minimized and converted to the Protein Data Bank (PDB) file format using the Molegro Virtual Docker (MVD) [8]. Aβ1-42 (PDB ID: 2BEG) was downloaded A. A. Mamun et al. 39 Figure 1. The ligand molecules: thioflavin T (ThT) and madecassoside (MD). from the PDB as a receptor for ThT and madecassoside docking. 2BEG is a 3D nuclear magnetic resonance solution structure of Aβ1-42, comprising residues 18 - 42 of a β-strand-turn-β-strand motif, and contains two intermolecular β-sheets that are formed by residues 18 - 26 (β 1) and 31 - 42 (β 2). 2BEG is a homopentamer, namely composed of A, B, C, D, and E monomer of Aβ1-42. Each monomer of 2BEG comprises 10 coordinate models [9]. The coordinates of model 1 of A monomer (A1), model 1 of B monomer (B1), and model 1 of C monomer (C1) were split from the composite 2BEG PDB file by MVD. Subsequently, the dimer, A1B1, was generated by Rossetta Dock [10] based on energy minimization. A trimer, A1B1C1, was also generated for madecassoside docking. 2.3. Molecular Docking of ThT and Madecassoside onto Aβ1-42 Dimer and Trimer 2.3.1. Docking with MVD The docking simulation was performed using the docking software MVD [8]. MVD is an automated docking software program with fast processing that automatically adds the missing hydrogen atoms of the ligand and receptor molecules, if any. The software also has a module to create a surface over the receptor molecule and identify potential binding sites for its activity. The program gives 10 conformational positions or so-called poses for the ligand and returns the five best poses with Mole Dock Score (equivalent to energy of binding/docking energy) and other thermodynamically calculated values. MVD also presents hydrogen bond information together with other thermodynamic values that suggest the formation of stable complexes between ligands and receptor molecules. MVD performs flexible ligand docking with optimization of the ligand geometry during docking. 2.3.2. Docking with Patch Dock Patch Dock [11] is a geometry-based molecular docking algorithm designed to find docking transformations that yield good molecular shape complementarity. Such transformations, when applied, induce both wide interface areas and small amounts of steric clashes. A wide interface ensures the inclusion of several matched local features of docked molecules with complementary characteristics. 3. Results 3.1. Molegro Virtual Docker (MVD) Docking performed by MVD provided the five best poses with corresponding Mol Dock score values and other thermodynamically calculated values. The 3D structures of bests coring dockings are shown in Figures 2(a)-(c). Steric interactions of both ThT and madecassoside, as ligands, with Aβ1-42 (the receptor) were also evaluated A. A. Mamun et al. 40 Figure 2. The best docking poses of the ligands, thioflavin T (ThT) and madecasoside (MD) with the receptors (Aβ1-42). ThT in pose 1 docked onto the A1B1 dimer (a), MD in pose 1 docked on toA1B1 dimer (b) and A1B1C1 trimer (c). Each monomeric strand, consisting of in-register parallel β1- and β2-sheets, runs perpendicularly along the long axis of the fiber. Interaction (binding) energies (−ΔG), shown as MolDock scores, are shown in (a)-(c). (a1)-(c1): contact (binding) maps of the interactions between atoms of ThT/MD and amino acid residues of the dimer/trimer (see also Table 1). Contact maps were visualized by Molegro Virtual Docker (MVD). Blue lines, formation of hydrogen bonds; red lines, steric interactions. A1, B1, and C1 are the corresponding coordinates of model 1 for the A, B, and C monomers of Aβ1-42, respectively. with MVD. Maps of the interactions of ThT with amino acid residues of the Aβ1-42 dimer (A1B1) are shown in Figure 2(a1). The docking of madecassoside to trimeric Aβ1-42 (A1B1C1) and its interaction map are shown in Figure 2(c1). The binding of madecassoside to the dimer was stronger than that of the trimer, as demonstrated by decreased binding energies (Figures 2(b)-(c)). Amino acids involved in the docking of madecassoside to the dimer (A1B1) and trimer (A1B1C1) were visualized by contact maps of the interactions between atoms of madecassoside and the amyloid amino acid residues using MVD (Figures 2(b1)-(c1), and Table 1). The amino acid residues of the A1B1 dimer found to sterically interact with ThT were Asp23, Gly33, and Leu34 of the A1 monomer and Ile32, Gly33, and Leu34 of the B1 monomer (Figures 2(a)-(a1)). Madecassoside sterically interacted with Glu22, Asp23, Gly33, Leu34 and Met35 of the A1 monomer, and Asp23, Lys28, Ile32, Gly33, Leu34 of the B1 monomer. These results clearly indicate that ThT and madecassoside have common binding sites at A. A. Mamun et al. 41 Table 1. Interaction sites of thioflavin T (ThT) and madecassoside (MD) with the amino acid residues of amyloid dimer (A1B1) and trimmer (A1B1C1). Amino acid residues Ala Glu Asp Val Gly Ser Asn Lys Gly Ala Ile Ile Gly Leu Met Val Gly Gly Val Val Ile Ala 1 - 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 ThT Dimer A1 B1 MD Dimer A1 B1 Trimer A1 B1 C1 Asp23 of the β1-sheet and Gly33 and Leu34 of the β2-sheet of the A1 monomer. These two ligands also had identical binding sites for the B1 monomer. In addition, the higher free energy of binding of madecassoside than ThT indicates stronger binding of madecassoside to the dimer (A1B1). Madecassoside was also found to bind with the amyloid trimer (A1B1C1); however, the binding energy was lower than that observed for the binding of madecassoside to the dimer (A1B1). 3.2. Patch Docker After performing molecular docking with MVD, we performed molecular docking of ThT and madecassoside using Patch Dock to confirm the ability of madecassoside to bind amyloid A1B1 dimer and A1B1C1 trimer (Figure 3). The algorithm used in Patch Dock performs rigid docking, with surface variability/flexibility implicitly addressed through liberal intermolecular penetration. Geometric shape complementarity scores were higher for madecassoside than for ThT when docked with both the dimer and the trimer. Approximate complex interface areas (receptor-ligand) and atomic contact energies were also higher for madecassoside (Table 2), suggesting stronger binding of madecassoside to the A1B1 dimer and A1B1C1 trimer than that of ThT. 4. Discussion Aβ1-42 peptides are important drug development targets because of their crucial role in neuronal diseases, including AD. Thus, the design of drugs that disrupt the formation of Aβ1-42-Aβ1-42 interfaces is particularly important for the development of therapies for the inhibition of Aβ1-42 fiber formation and/or the dismantling of preformed Aβ1-42 fibers. In other words, the development of Aβ1-42 fibrillation antagonists may provide an increased understanding of the pathogenesis of AD, leading to the development of more effective therapies. We previously demonstrated that co-incubation of free Aβ1-42 monomers with madecassoside prevented fiber polymerization [5]. The inhibitory effect of madecassoside on Aβ1-42 fibril formation corroborated previous ThT fluorescence spectroscopy and transmission electron microscopy findings [6]. In this study, our intention was to computationally model the interaction of madecassoside with Aβ1-42 by molecular docking. Ligand binding sites for Aβ1-42 were examined using high-resolution 3D coordinates obtained from the PDB. Finally, we sought to obtain information regarding the mechanism of madecassoside-induced inhibition of amyloid fibril formation by computationally modeling the binding of ThT with Aβ1-42. We previously reported that Aβ fibers bind to ThT [12]-[15]. ThT is one of the most widely used amyloid dyes used in the hundreds of amyloid studies published every year. The intensive use of ThT as an in vitro marker of amyloid formation has provided substantial information regarding the mechanism of ThT binding. Further studies aim to fully determine common structure(s) of the amyloid peptide recognized by ThT. Within the past several years, a series of critical experimental analyses of ThT have elucidated, in detail, many of the atomiclevel interactions underlying ThT binding and fluorescence. Because several recent reports have discussed the broad range of amyloid staining dyes [16]-[18], in the present study, we instead focused on the atomiclevel mechanisms underlying the binding of ThT to amyloid fibrils. Amyloid fibrils may have a specific ThT binding site that sterically “locks” the bound dye, leading to the enhancement of ThT fluorescence. These properties of ThT made it an ideal positive reference compound for this A. A. Mamun et al. 42 Figure 3. The best scoring results of the Patch Docking of Thioflavin T (ThT) (a)-(b) and madecassoside (MD) (c)-(d) with theA1B1 dimer and A1B1C1 trimer. Ligands (ThT and MD) are represented by green surface-fill representation and the receptors (A1B1 dimer and A1B1C1 trimer) are in ribbons + surface presentation. Table 2. Patch docking of thioflavin T (ThT) and madecassoside (MD) with the amyloid dimer (A1B1) and trimer (A1B1C1). Docking (Receptor vs. Ligands) Score Area ACE A1B1 dimer vs. ThT 2942 356.20 −314.68 A1B1 dimer vs. MD 6130 789.50 −470.03 A1B1C1 trimer vs. ThT 2820 307.50 −261.45 A1B1C1 trimer vs. MD 6586 845.50 −460.37 Score: Geometric shape complementarity score; Area: Approximate interface area of the complex (receptor-ligand); ACE: Atomic contact energy; A1B1 dimer: Generated by feeding the A1 and B1 model 1 of 2BEG to Rosetta Server; A1B1C1 trimer: Generated feeding the A1, B1 and C1 coordinate model 1 of 2BEG; ThT:Thioflavin T and MD: madecassoside. study. Structure-based drug design has made substantial contributions to drug discovery, including the development of treatments for cancer and drug-resistant infectious diseases. Computational structure-based drug design may, therefore, facilitate the development of novel treatments for AD. We recently reported that asiaticoside, another polyphenol compound of Centella asiatica, also inhibited fibril formation [19]. Therefore, the docking of madecassoside with the Aβ1-42 dimer/trimer was used to corroborate our previous findings regarding the in vivo and in vitro inhibitory effects of madecassoside on fibril formation and memory impairment in an AD rat model [6]. Aggregation-prone amino acids, i.e., hotspots for Aβ1-42 aggregation, are located at residues 17 - 22 and 32 - 42 [19]. While docked with the dimer/trimer, madecassoside displayed greater binding affinity, according to binding energy, than ThT. These results demonstrate that madecassoside is capable of binding amyloid fibers, potentially providing an explanation for the reduced amyloid burden observed in the brains of madecassoside-administered AD model rats and concurrent inhibition of in vitro fibril formation [6]. Notably, residues 17 - 21 and residues 31 - 42 are β-sheet forming amino acids engaged in monomer-monomer (dimer) inter surface interactions [19]. The common features between MVD and Patch Docke were displayed in their common amino acid binding sites for madecassoside: Asp23 and Leu34 of the Aβ1-42 (analysis not shown). These results again suggest that inter surface interaction sites is the most promising target sites for ligands that potentially inhibit amyloid fibril interactions, such as madecassoside. Hossain et al. (2009) previously reported that the dimeric/trimeric form of Aβ1-42 acts as one of the seeding units for Aβ1-42 fibril formation [12]. Therefore, we assessed the molecular docking of the dimeric/trimeric structures of Aβ1-42 with madecassoside. Polyphenols are capable of forming hydrogen bonds with amyloids and other proteins [20] [21]. In the present in silico study, madecassoside was A. A. Mamun et al. 43 found to affect Aβ1-42 fibril formation through binding to amino acid residues 17 - 21 and 31 - 42, the most aggregation-sensitive regions of Aβ1-42, and forming hydrogen bonds with Aβ1-42. The neurotoxicity of Aβ1-42 involves the in vivo conformational transition from soluble α-helical to insoluble β-sheet forms of the peptide following release from the cell membrane into the surrounding aqueous environment. 5. Conclusion Herein, we demonstrate the successful molecular docking of madecassoside on to Aβ1-42, with a higher affinity of binding than that with the thioflavin T, consistent with the inhibitory effects of madecassoside on in vitro fibril formation and memory impairment in a rat model of AD. Our in silico results further provide compelling evidence for the utility of triterpene glycosides as a preventive medication for neurodegenerative diseases such as Aβ1-42 aggregation-induced AD. Acknowledgements This work was supported in part by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education Culture, Sports, Science and Technology, Japan. The authors would like to thank Enago (www.enago.jp) for the English language review. References [1] Selkoe, D.J. (1991) The Molecular Pathology of Alzheimer’s Disease. Neuron, 6, 487-498. http://dx.doi.org/10.1016/0896-6273(91)90052-2 [2] Iwatsubo, T., Odaka, A., Suzuki, N., Mizusawa, H., Nukina, N. and Ihara, Y. (1994) Visualization of Aβ 42(43) and Aβ40 in Senile Plaques with End-Specific Aβ Monoclonals: Evidence That an Initially Deposited Species Is Aβ 42(43). Neuron, 13, 45-53. http://dx.doi.org/10.1016/0896-6273(94)90458-8 [3] Ferri, C.P., Prince, M., Brayne, C., Brodaty, H., Fratiglioni, L., Ganguli, M., Hall, K., Hasegawa, K., Hendrie, H., Huang, Y.Q., Jorm, A., Mathers, C., Menezes, P.R., Rimmer, E. and Scazufca, M. (2005) Global Prevalence of Dementia: A Delphi Consensus Study. The Lancet, 366, 2112-2117. http://dx.doi.org/10.1016/S0140-6736(05)67889-0 [4] Wanasuntronwong, A., Tantisira, M.H., Tantisira, B. and Watanabe, H. (2012) Anxiolytic Effects of Standardized Extract of Centella asiatica (ECa 233) after Chronic Immobilization Stress in Mice. Journal of Ethnopharmacology, 143, 579-585. http://dx.doi.org/10.1016/j.jep.2012.07.010 [5] Hossain, S., Hashimoto, M., Katakura, M. and Shido, O. (2013) Asiaticoside and Madecassoside, Two Major Glycosides of Centella asiatica Inhibit the in Vitro Amyloid Beta Peptide Aβ1-42 Fibrillation—Assessed by FCS, Capable of Detecting Single Molecular Movement and Interaction. Proceedings of the 11th International Conference on Alzheimer’s & Parkinson’s Diseases, Florence, 6-10 March 2013, 245. [6] Mamun, A.A., Hashimoto, M., Katakura, M., Matsuzaki, K., Hossain, S., Arai, H. and Shido, O. (2014) Neuroprotective Effect of Madecassoside Evaluated Using Amyloid β1-42-Mediated in Vitro and in Vivo Alzheimer’s Disease Models. International Journal of Indigenous Medicinal Plants, 47, 1669-1682. [7] Marvin (2011) Marvin Was Used for Drawing, Displaying and Characterizing Chemical Structures, Substructures and Reactions, Marvin 5.7. [8] Thomsen, R. and Christensen, M.H. (2006) MolDock: A New Technique for High-Accuracy Molecular Docking. Journal of Medicinal Chemistry, 49, 3315-3321. http://dx.doi.org/10.1021/jm051197e [9] Lührs, T., Ritter, C., Adrian, M., Riek-Loher, D., Bohrmann, B., Döbeli, H., Schubert, D. and Riek, R. (2005) 3D Structure of Alzheimer’s Amyloid-Beta (1-42) Fibrils. roceedings of the National Academy of Sciences of the United States of America, 102, 17342-17347. http://dx.doi.org/10.1073/pnas.0506723102 [10] Lyskov, S. and Gray, J.J. (2008) The RosettaDock Server for Local Protein-Protein Docking. Nucleic Acids Research, 36, W233-W238. http://dx.doi.org/10.1093/nar/gkn216 [11] Duhovny, D., Nussinov, R. and Wolfson, H.J. (2002) Efficient Unbound Docking of Rigid Molecules. In: Guigó, R. and Gusfield, D., Eds., Proceedings of the 2nd Workshop on Algorithms in Bioinformatics (WABI) Rome, Italy, Lecture Notes in Computer Science (LNCS) 2452, Springer Verlag, Berlin Heidelberg, 185-200. [12] Hossain, S., Hashimoto, M., Katakura, M., Miwa, K., Shimada, T. and Shido, O. (2009) Mechanism of Docosahexaenoic Acid-Induced Inhibition of in Vitro Aβ1−42 Fibrillation and Aβ1−42-Induced Toxicity in SH-S5Y5 Cells. Journal of Neurochemistry, 111, 568-579. http://dx.doi.org/10.1111/j.1471-4159.2009.06336.x [13] Hashimoto, M., Hossain, S., Yamashita, S., Katakura, M., Tanabe, Y., Fujiwara, H., Gamoh, S., Miyazawa, T., Arai, H., Shimada, T. and Shido, O. (2008) Docosahexaenoic Acid Disrupts in Vitro Amyloid β1−40 Fibrillation and Conco- A. A. Mamun et al. 44 mitantly Inhibits Amyloid Levels in Cerebral Cortex of Alzheimer’s Disease Model Rats. Journal of Neurochemistry, 107, 1634-1646. http://dx.doi.org/10.1111/j.1471-4159.2008.05731.x [14] Hashimoto, M., Hossain, S., Katakura, M., Mamun, A.A. and Shido, O. (2015) The Binding of Aβ1−42 to Lipid Rafts of RBC Is Enhanced by Dietary Docosahexaenoic Acid in Rats: Implicates to Alzheimer’s Disease. Biochimica et Biophysica Acta, 1848, 1402-1409. http://dx.doi.org/10.1016/j.bbamem.2015.03.008 [15] Hashimoto, M., Hossain, S., Hossain, S., Rahman, A., Shimada, T. and Shido, O. (2011) Docosahexaenoic Acid Withstands the Aβ25-35-Induced Neurotoxicity in SH-SY5Y Cells. The Journal of Nutritional Biochemistry, 22, 22-29. http://dx.doi.org/10.1016/j.jnutbio.2009.11.005 [16] Groenning, M. (2009) Binding Mode of Thioflavin T and Other Molecular Probes in the Context of Amyloid FibrilsCurrent Status. Journal of Chemical Biology, 3, 1-18. http://dx.doi.org/10.1007/s12154-009-0027-5 [17] Kim, Y., Lee, J.H., Ryu, J. and Kim, D.J. (2009) Multivalent & Multifunctional Ligands to Beta-Amyloid. Current Pharmaceutical Design, 15, 637-658. http://dx.doi.org/10.2174/138161209787315648 [18] Nilsson, K.P. (2009) Small Organic Probes as Amyloid Specific Ligands-Past and Recent Molecular Scaffolds. FEBS Letters, 583, 2593-2599. http://dx.doi.org/10.1016/j.febslet.2009.04.016 [19] Hossain, S., Hashimoto, M., Katakura, M., Mamun, A.A. and Shido, O. (2015) Medicinal Value of Asiaticoside for Alzheimer’s Disease as Assessed Using Single-Molecule-Detection Fluorescence Correlation Spectroscopy, LaserScanning Microscopy, Transmission Electron Microscopy, and in Silico Docking. BMC Complementary and Alternative Medicine, 15, in Press. http://dx.doi.org/10.1186/s12906-015-0620-9 [20] Sirk, T.W., Friedman, M. and Brown, E.F. (2011) Molecular Binding of Black Tea Theaflavins to Biological Membranes: Relationship to Bioactivities. Journal of Agricultural and Food Chemistry, 59, 3780-3787. http://dx.doi.org/10.1021/jf2006547 [21] Stefani, M.S. (2013) Protein Folding and Aggregation into Amyloid: The Interference by Natural Phenolic Compounds. International Journal of Molecular Sciences, 14, 12411-12457. http://dx.doi.org/10.3390/ijms140612411