
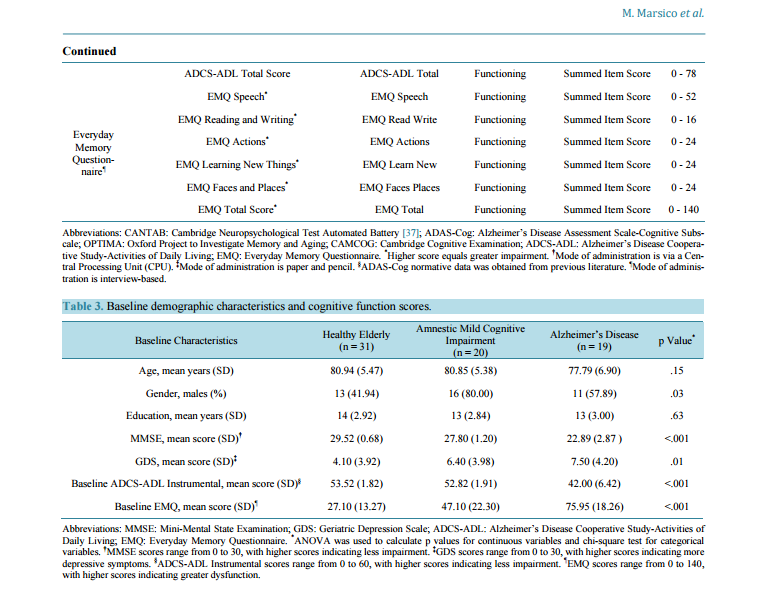
 Cognitive and Functional Profiles in Mild-to-Moderate Alzheimer’s Disease and Mild Cognitive Impairment Compared to Healthy Elderly Mark Marsico1*, Celeste A. de Jager2,3, April Grant1, Xingshu Zhu4, Arwen Markwick2, Julie Chandler1 1 Epidemiology Department, Merck Research Laboratories, North Wales, USA 2 OPTIMA, Nuffield Department of Medicine, University of Oxford, Oxford, UK 3 Division of Geriatric Medicine, Department of Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa 4 Statistical Programming, Merck Research Laboratories, North Wales, USA Email: * mark.marsico@merck.com Received 24 October 2014; revised 26 November 2014; accepted 9 December 2014 Academic Editor: Lei Xue, School of Life Science, Tongji University, China Copyright © 2014 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Background: Amnestic mild cognitive impairment (aMCI) and mild-to-moderate Alzheimer’s disease (AD) are clinically distinct but impact cognitive and functional ability similarly. Comprehensive assessment of cognitive and functional deficits may prove useful in informing differential diagnosis in early stages of dementia and in informing endpoint selection in therapeutic AD trials. Objective: The objective of this study was to characterize patterns of cognitive and functional impairment in aMCI and mild-to-moderate AD subjects compared to cognitively intact healthy elderly (HE). Methods: Thirty-one healthy elderly, 20 aMCI and 19 AD participants were administered a cognitive test battery that included the ADAS-Cog and functional assessments. Z-scores were calculated for all endpoints based on the HE reference group. Results: Cognitive deficits were observed in AD and aMCI participants relative to the referent group. On average, aMCI participants performed 1 - 2 standard deviations below HE on cognitive tests, and AD participants performed 2 - 3 standard deviations below HE. Domain-specific functional deficits among AD participants (zscore −0.4 to −6.4) were consistently greater than those of aMCI participants (z-score 0 to −1.7). Conclusion: This study provides further support for comprehensive assessment and monitoring of * Corresponding author. M. Marsico et al. 169 cognitive and functional domain scores in the diagnosis and treatment of aMCI and mild AD. Domain-specific cognitive scores may be more useful than composite scores in characterizing impairment and decline. Measuring domains such as attention, processing speed and executive function may increase the sensitivity of detecting disease progression and therapeutic effects, particularly in mild-moderate AD where memory decline may be too slow to detect drug effects during a typical clinical trial. Keywords Alzheimer’s Disease, Amnestic Mild Cognitive Impairment, Dementia, Cognition 1. Introduction Mild Cognitive Impairment (MCI) is characterized by changes in cognition which are less severe and widespread than those of Alzheimer’s disease (AD), but represent a decline from cognitive functioning of normal aging and predisposes one to the risk of developing AD [1]. Initially, recognition of MCI was identified by detection of memory performance below age-associated norms [2]. However, recent revisions of this syndrome acknowledge that deficits may also occur in other cognitive domains [3]. Cognitive decline is known to be preceded by neuropathology including beta-amyloid plaque deposition, neurofibrillary tangle formation and atrophy in the brain. Novel research with neuroimaging and CSF markers of pathology has led to revised research criteria for AD diagnosis [4]. The criteria include memory impairment as assessed with sensitive episodic memory tests. This new diagnostic framework has stimulated debate about the definition of AD and related conditions. The Mini-Mental State Examination (MMSE) and Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog) have typically been used as outcome measures in AD drug intervention trials [5]; however, these measures may be insensitive for detecting early and subclinical changes [6]. It is widely accepted that the typical cognitive profile in early AD is marked by episodic memory impairment with semantic memory, working memory, executive functioning, and visuospatial and attentional dysfunction emerging as the disease progresses [7]-[9]. The heterogeneity of cognitive decline suggests endpoints that comprehensively assess cognition may be best suited for detecting cognitive change [10]-[12]. AD is also marked by deficits in functional ability, characterized by progressive deterioration of activities of daily living (ADL), including early decline in instrumental activities, and later activities relating to basic selfcare [13]. Once loss of ADL occurs and independent living is problematic, diagnosis will include dementia. ADL impairments associated with MCI may occur for instrumental activities that require high-level cognitive skills [14] [15] such as use of technology, understanding cultural expectations [16], managing money [17], medication use [18] and executive functioning [13]. As with cognitive measures, indications of differential profiles of functional impairment have also been found for AD as compared to other dementia groups [19]. Countless cognitive tests have been used worldwide [20] to assess cognitive change in observational studies and clinical trials. In many instances, cognitive tests are included alongside the MMSE and ADAS-Cog in order to evaluate the potential utility of the measures as compared to these “gold standard” trial endpoints. An objective of the Oxford Project to Investigate Memory and Aging (OPTIMA) study was to identify cognitive tests capable of discriminating between cognitively healthy and cognitively impaired groups [21]-[23] as a first step in identifying the tests most likely to identify preclinical AD and to aid in diagnosis of dementia types. For this study, an adjunct to the original OPTIMA cohort, a selection of cognitive measures was added to the OPTIMA battery with the objective of identifying psychometrically sound cognitive measures for use as cognition endpoints in future AD clinical trials. The purpose of this analysis was to determine, in a single cohort of participants, the pattern of cognitive and functional impairment of amnestic MCI-subtype (aMCI) and AD participants, using scores from HE as a reference. Clarification of cognitive and functional profiles is important for accurate differential diagnosis and monitoring decline across dementia syndromes [8] [24]-[27]. Furthermore, comprehensive understanding of domainspecific cognitive and functional profiles in aMCI and AD is the first step in identifying particular measures that are sensitive to impairment and the domains that can be utilized as endpoints to better characterize disease and assess impact on the early treatment to mild AD in clinical trials. M. Marsico et al. 170 2. Materials and Methods 2.1. Participants Participants were recruited from a larger cohort of living subjects enrolled in the Oxford Project to Investigate Memory and Aging (OPTIMA) study within the United Kingdom [28], a longitudinal study of memory and aging established at the University of Oxford. All enrolled subjects undergo a clinical and informant-based interview, a comprehensive cognitive assessment including the Cambridge Cognitive Examination (CAMCOG) [29] [30] and ADAS-Cog, physical examination, MRI brain scan and blood-screening tests as part of the scheduled protocol for diagnostic purposes. Potential participants for the current prospective study were identified from the OPTIMA participant database according to clinical diagnosis at last visit. Those with Mini-Mental State Examination (MMSE) [31] score of ≥16 (N = 224) had their medical records screened for inclusion/exclusion criteria and rescreened to assess suitability for inclusion in the study. Sixty one subjects were excluded (27%) based on a review of their medical record. A further 73 (33%) were excluded after not responding to study invitation letters and telephone calls. The most common reasons for exclusion based on the chart review and telephone screen were: acute illnesses, recent stroke, advanced cancer, other dementia diagnosis than AD, advanced dementia or unwillingness to participate. The remaining 90 subjects (40%) were screened by telephone. Current dementia medication and administration of the Telephone Interview for Cognitive Status-Modified (TICS-M) [32] were used to establish a preliminary diagnostic classification. General inclusion criteria applicable to all subjects for this sub-study, except where noted, were 60 years of age or older, no medical condition affecting cognition (excluding AD), a reliable informant willing to act as a study partner (not required for HE), adequate sensory and motor capabilities to perform cognitive testing, fluency in English as determined by the investigator; and the ability to understand study procedures and give verbal consent to participate. Exclusions included: a history of disease that might confound study results, a significant psychiatric history, recent (within 2 years) or current evidence of major untreated depressive or psychiatric disorder, uncontrolled or untreated endocrine disease or other medical condition causing transient or continuous alteration of consciousness or attention, recent (within 2 years) or current evidence of major stroke, multiple lacunar infarcts, transient ischemic events (within 3 months), epilepsy, Parkinson’s disease, progressive supranuclear palsy, Huntington’s disease, amyotrophic lateral sclerosis, multiple sclerosis or other central neurological disorder or a history of significant head trauma with loss of consciousness. Final diagnostic classification was made at a consensus meeting of at least two research staff consisting of neuropsychologists and nurses. Cognitive and functional cohort specific diagnostic classification criteria are provided in Table 1. 2.2. Procedures In addition to screening and diagnostic assessments (i.e. Clinical Dementia Rating (CDR) [33], MMSE, Subjective Memory Complaint (SMC) [30] and Geriatric Depression Scale (GDS) [34]), eligible subjects underwent cognitive testing and completed functional and self- and informant-reported assessments at the screening visit. Cognitive and functional data collected at this visit were considered “baseline” data. At subsequent study visits, administrations of cognitive and functional measures were repeated. Information about changes in subject health Table 1. Cognitive and functional diagnostic classification criteria. HE aMCI AD • CDR = 0 • MMSE ≥ 28 • GDS < 20 • TICS-M total score ≥ 29 • TICS-m WLR ≥ 11 • No significant SMC • Without evidence of depression • Without dementia of ADL impairment • CDR ≤ 0.5 • MMSE ≥ 23 • GDS < 20 • TICS-M total score < 29 and > 14 • TICS-m WLR < 10 • Without dementia and with minimal or no ADL impairment • Met Petersen criteria including the presence of SMC • CDR > 0.5 • MMSE ≥ 16 • GDS < 20 • TICS-M total score < 29 and > 14 • TICS-m WLR < 10 • Possible or probable AD according to previous or new clinical diagnosis using the NINCDS-ADRDA criteria Abbreviations: CDR: Clinical Dementia Rating; MMSE: Mini-Mental State Examination; GDS: Geriatric Depression Scale; TICS-M: Telephone Interview for Cognitive Status-Modified; WLR: Word List Recall; SMC: Subjective Memory Complaint; ADL: Activities of Daily Living; NINCDSADRDA: National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association. M. Marsico et al. 171 or medical conditions, including changes in medication use and medical events occurring between visits, was collected. The structure of study visits and sequence of study assessments were consistent for all subjects. All study procedures and assessments were administered by either trained research nurses or research neuropsychologists. Only the study’s baseline, cross-sectional data are presented in this paper. Longitudinal data collected across subsequent visits (6 and 12 months after baseline) are not presented. Subjects were not administered any marketed or investigational compound in this study. Subjects were however, undergoing usual care by their healthcare providers for their disease which may have included medication indicated for the treatment of AD. Because most subjects began their AD treatment prior to their inclusion in the study, responsiveness (i.e. the ability of a measure to detect pharmacological change) could not be assessed. All study procedures were in accord with the ethical standards of the Committee on Human Experimentation of the institution in which the experiments were done or in accord with the Helsinki Declaration of 1975. Informed consent was taken on recruitment into the OPTIMA cohort. Ethical approval for this study was granted by the Frenchay Regional Ethics Committee, reference 09/H0107/09. 2.3. Measures Cognitive tests were chosen for this study based on published evidence of the tests’ psychometric properties and their usefulness in assessing cognition in AD and aMCI [35]. Tests sensitive to mild impairment were chosen for comparison with the broadly used ADAS-Cog. The overarching aim in selecting cognitive measures was to achieve broad domain coverage with at least two measures assessing each cognitive domain known to be impacted in AD. The cognitive test battery included the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADASCog) [36]; the Cambridge Automated Neuropsychological Test Battery (CANTAB) [37] Paired Associate Learning (PAL), Reaction Time Index (RTI) and Spatial Working Memory tests; the CAMCOG; and domainspecific tests in verbal and visuospatial episodic memory, semantic memory, attention, executive function and information processing speed (see Table 2). Functional measures included the Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL) [38], scored on basic and instrumental ADL, and the Everyday Memory Questionnaire (EMQ) [39]. Detailed description of all study measures can be found in the online supplement. 2.4. Statistical Methods Standardized z-scores were calculated for all cognitive and functional baseline data based on the mean and standard deviation scores in the HE group. Standardizing the aMCI and mild-to-moderate AD performance to a z-score based on a HE sample allows all test and domain scores to be reported based on the same scale with mean of 0 and a standard deviation of 1. Domain composite scores were calculated by summing the individual tests’ z-score means and dividing by the number of tests comprising that domain. Individual cognitive tests were grouped according to domain as indicated in Table 2. The effects of age and education were assessed using one-way analysis of variance (ANOVA); independent t-tests were conducted to investigate the potential influence of gender. A p-value of <0.05 was considered significant for all comparisons. Because cognitive performance was not impacted by any of these covariates (data not shown), unadjusted, raw data were used in all z-score calculations. Because the ADAS-Cog was not administered to HE in this study, standard scores were created using previously published normative data [40] [41]. This study was designed to descriptively assess cognitive performance and functional status in non-demented, aMCI and mild-to-moderate AD. The study was not powered to support formal statistical testing comparing the cognitive and functional outcomes across the three groups. However, statistical tests (ANOVA) were performed and p-values presented to facilitate interpretation of the differences between groups with regard to their cognitive and functional scores. Imputation was not performed for missing data so the number of subjects contributing to group means may vary by endpoint. All statistical analyses were performed using SAS version 9.3. 3. Results 3.1. Demographic Characteristics Seventy five OPTIMA subjects prescreened with the TICS-M were invited to come into the study clinic for a M. Marsico et al. 172 Table 2. Cognitive and functional test characteristics. Cognitive/ Functional Battery Test Name Abbreviation Cognitive Domain Outcome Variables Range CANTAB Computerized Battery† CANTAB Reaction Time 5-Choice Movement Time* 5cRT Movement Attention & Processing Milliseconds 0 - ∞ CANTAB Reaction Time 5-Choice Reaction Time* 5cRT Reaction Attention & Processing Milliseconds 0 - ∞ CANTAB Reaction Time Simple Movement Time* sRT Movement Attention & Processing Milliseconds 0 - ∞ CANTAB Reaction Time Simple Reaction Time* sRT Reaction Attention & Processing Milliseconds 0 - ∞ CANTAB Paired Associates Learning Total Errors* PALTOTEA Visuospatial Memory Total Errors Adjusted 0 - 158 CANTAB Spatial Working Memory Between Errors* SWMBE Working Memory Errors 0 - 326 ADAS-Cog and Subtests‡,§ ADAS-Cog Total Score* ADAS-Cog Total General Cognition Total Errors 0 - 70 ADAS-Cog Naming Objects and Fingers* ADAS-cog Objects Semantic Memory Total Incorrect 0 - 17 ADAS-Cog Word Recall* ADAS-cog Recall Verbal Episodic Memory Mean Words per Trial Not Recalled 0 - 10 ADAS-Cog Word Recognition* ADAS-Cog Recognition Verbal Episodic Memory Words Not Recognized 0 - 24 Mazes Seconds* Mazes Executive Function Seconds 0 - 240 OPTIMA‡ Hopkins Verbal Learning Test (Total Recall) HVLT TR Verbal Episodic Memory Words Recalled 0 - 36 Hopkins Verbal Learning Test (Delayed Recall) HVLT DR Verbal Episodic Memory Words Recalled 0 - 12 The Placing Test TPT Objects Visuospatial Memory Number Correct 0 - 10 CAMCOG Learning Learn Episodic Memory Number Correct 0 - 17 CAMCOG Recent Memory Recent Episodic Memory Number Correct 0 - 4 CAMCOG Remote Memory Remote Episodic Memory Number Correct 0 - 6 CAMCOG Comprehension Comprehension Semantic Memory Summed Ordinal Scale 0 - 9 CAMCOG Expression Expression Semantic Memory Summed Ordinal Scale 0 - 21 Graded Naming Test GNT Semantic Memory Number Correct 0 - 30 CAMCOG Calculation Calculation Working Memory Number Correct 0 - 2 Symbol Digit Modalities Test SDMT Executive Function Completed Minus Errors 0 - 110 Clock Drawing Task 1 CLOX 1 Executive Function Number Correct 0 - 15 CAMCOG Abstract Thinking Thinking Executive Function Summed Ordinal Scale 0 - 8 CAMCOG Orientation Orientation Orientation Number Correct 0 - 10 CAMCOG Perception Perception Perception Number Correct 0 - 11 CAMCOG Praxis Praxis Praxis Number Correct 0 - 12 CAMCOG Attention Attention Attention & Processing Accuracy & Summed Scale 0 - 7 Map Search from the Tests for Everyday Attention MAP Search Attention & Processing Items Circled 0 - 80 Letter Comparison Speed LCS Processing Speed Accuracy Score 0 - 20 Pattern Comparison Speed PCS Processing Speed Accuracy Score 0 - 30 Cambridge Cognitive Examination (CAMCOG total score) CAMCOG Total General Cognition Subscales Total Score 0 - 107 ADCS-ADL¶ ADCS-ADL Basic Activities of Daily Living Basic ADL Functioning Summed Item Score 0 - 18 ADCS-ADL Instrumental Activities of Daily Living Instrumental ADL Functioning Summed Item Score 0 - 60 M. Marsico et al. 173 Continued ADCS-ADL Total Score ADCS-ADL Total Functioning Summed Item Score 0 - 78 Everyday Memory Questionnaire¶ EMQ Speech* EMQ Speech Functioning Summed Item Score 0 - 52 EMQ Reading and Writing* EMQ Read Write Functioning Summed Item Score 0 - 16 EMQ Actions* EMQ Actions Functioning Summed Item Score 0 - 24 EMQ Learning New Things* EMQ Learn New Functioning Summed Item Score 0 - 24 EMQ Faces and Places* EMQ Faces Places Functioning Summed Item Score 0 - 24 EMQ Total Score* EMQ Total Functioning Summed Item Score 0 - 140 Abbreviations: CANTAB: Cambridge Neuropsychological Test Automated Battery [37]; ADAS-Cog: Alzheimer’s Disease Assessment Scale-Cognitive Subscale; OPTIMA: Oxford Project to Investigate Memory and Aging; CAMCOG: Cambridge Cognitive Examination; ADCS-ADL: Alzheimer’s Disease Cooperative Study-Activities of Daily Living; EMQ: Everyday Memory Questionnaire. * Higher score equals greater impairment. † Mode of administration is via a Central Processing Unit (CPU). ‡ Mode of administration is paper and pencil. § ADAS-Cog normative data was obtained from previous literature. ¶ Mode of administration is interview-based. Table 3. Baseline demographic characteristics and cognitive function scores. Baseline Characteristics Healthy Elderly (n = 31) Amnestic Mild Cognitive Impairment (n = 20) Alzheimer’s Disease (n = 19) p Value* Age, mean years (SD) 80.94 (5.47) 80.85 (5.38) 77.79 (6.90) .15 Gender, males (%) 13 (41.94) 16 (80.00) 11 (57.89) .03 Education, mean years (SD) 14 (2.92) 13 (2.84) 13 (3.00) .63 MMSE, mean score (SD)† 29.52 (0.68) 27.80 (1.20) 22.89 (2.87 ) <.001 GDS, mean score (SD)‡ 4.10 (3.92) 6.40 (3.98) 7.50 (4.20) .01 Baseline ADCS-ADL Instrumental, mean score (SD)§ 53.52 (1.82) 52.82 (1.91) 42.00 (6.42) <.001 Baseline EMQ, mean score (SD)¶ 27.10 (13.27) 47.10 (22.30) 75.95 (18.26) <.001 Abbreviations: MMSE: Mini-Mental State Examination; GDS: Geriatric Depression Scale; ADCS-ADL: Alzheimer’s Disease Cooperative Study-Activities of Daily Living; EMQ: Everyday Memory Questionnaire. * ANOVA was used to calculate p values for continuous variables and chi-square test for categorical variables. † MMSE scores range from 0 to 30, with higher scores indicating less impairment. ‡ GDS scores range from 0 to 30, with higher scores indicating more depressive symptoms. § ADCS-ADL Instrumental scores range from 0 to 60, with higher scores indicating less impairment. ¶ EMQ scores range from 0 to 140, with higher scores indicating greater dysfunction. baseline visit. Only 5 (7%) participants (all MCI or AD) failed to meet inclusion criteria. The final sample consisted of 31 HE, 20 aMCI and 19 AD. Results comparing demographic factors by group are presented in Table 3. On average, AD subjects had less education than the HE and were approximately 3 years younger than the HE and aMCI, but the differences were not statistically significant. There were significantly more males recruited into the aMCI and AD groups compared to HE. MMSE scores were statistically different across the groups with highest scores among the HE (29.5 ± 0.7) and aMCI (27.8 ± 1.2) compared to AD (22.9 ± 2.9). A gradient of increasing functional impairment and depressive symptoms with increasing cognitive impairment was observed between the groups. At baseline, 8 (42%) mild-to-moderate AD subjects had received cognitive-enhancing treatment as part of their usual medical care for an average of 3.9 ± 3.2 years. 3.2. Cognitive Performance All means and standard deviations for cognitive test performance in the mild-to-moderate AD, aMCI, and HE groups can be found in Table 4. Figure 1 presents the cross-sectional baseline z-scores for AD and aMCI, grouped by cognitive domain, for each of the cognitive endpoints evaluated. Cut-off lines of 1.5 and 2 standard deviations below the mean are indicated as the usual thresholds of significant deficit from normal control performance for MCI and AD, respectively [2]. All references made to the deficits observed in AD and aMCI groups are always relative to the HE referent group. Minimum and maximum z-scores within a cognitive do- M. Marsico et al. 174 Table 4. Means and standard deviations for cognitive and functional tests. Test Abbreviation N HE Mean ± SD N aMCI Mean ± SD N AD Mean ± SD 5cRT Movement* 31 311.32 ± 100.74 20 335.75 ± 117.27 17 425.76 ± 171.22 5cRT Reaction* 31 383.94 ± 51.94 20 466.10 ± 73.63‡ 17 491.71 ± 112.49 sRT Movement* 31 312.26 ± 158.18 20 311.75 ± 131.15 17 444.00 ± 213.33 sRT Reaction* 31 342.65 ± 65.17 20 431.70 ± 114.87 17 466.12 ± 254.93 PALTOTEA* 30 37.80 ± 23.93 19 74.53 ± 33.27‡ 18 117.94 ± 18.53 SWMBE* 31 62.84 ± 15.76 20 25.60 ± 13.00‡ 17 30.35 ± 5.59 ADAS-Cog Total* 107 5.6 ± 3.3† 20 11.38 ± 4.78 19 24.74 ± 10.40§ ADAS-Cog Objects* 124 0.05 ± 0.22† 20 1.10 ± 0.97 19 2.26 ± 2.21§ ADAS-Cog Recall* 107 2.7 ± 1.2† 20 4.53 ± 1.30 19 6.63 ± 1.60§ ADAS-Cog Recognition* 107 2.0 ± 2.2† 20 4.35 ± 3.15 19 8.16 ± 3.85§ Mazes* 107 30.0 ± 27.9† 20 33.00 ± 15.50 19 40.21 ± 16.51 HVLT TR 31 24.35 ± 4.32 19 19.05 ± 4.56‡ 19 11.32 ± 5.20§ HVLT DR 30 8.13 ± 2.29 19 5.79 ± 2.42‡ 19 0.05 ± 0.23§ TPT Objects 31 9.29 ± 0.90 19 8.58 ± 1.98 19 3.58 ± 1.87§ Learn 31 13.74 ± 1.79 19 11.26 ± 2.51‡ 13 4.38 ± 1.45§ Recent 31 3.97 ± 0.18 19 3.79 ± 0.54 13 1.92 ± 1.04§ Remote 31 5.45 ± 0.62 19 5.11 ± 0.88 13 3.38 ± 0.96§ Comprehension 31 8.94 ± 0.25 19 8.74 ± 0.56 13 8.31 ± 0.63§ Expression 31 19.52 ± 1.31 19 17.82 ± 1.07‡ 13 16.38 ± 2.87 GNT 31 26.35 ± 3.22 19 23.26 ± 4.17 19 14.05 ± 8.39§ Calculation 31 1.97 ± 0.18 19 1.79 ± 0.54 13 1.54 ± 0.66 SDMT 12 40.33 ± 9.59 11 30.91 ± 10.63 18 24.06 ± 11.83 CLOX 1 5 14.20 ± 0.45 9 12.22 ± 1.72 12 9.67 ± 3.87 Thinking 31 7.68 ± 0.60 19 7.16 ± 1.01 13 5.85 ± 2.12§ Orientation 31 9.97 ± 0.18 19 9.53 ± 0.70 13 6.46 ± 1.85§ Perception 31 10.42 ± 0.85 19 10.32 ± 1.16 13 8.54 ± 2.03§ Praxis 31 11.68 ± 0.48 19 10.89 ± 1.15‡ 13 10.62 ± 1.12 Attention 31 6.68 ± 0.79 19 6.89 ± 0.32 13 6.15 ± 1.07§ MAP Search 31 52.29 ± 13.86 18 43.72 ± 14.93 19 30.05 ± 16.02§ LCS 10 7.70 ± 2.50 10 7.30 ± 2.06 15 5.60 ± 2.13 PCS 10 11.60 ± 1.51 9 11.78 ± 2.59 15 8.93 ± 2.31§ CAMCOG Total 31 100.00 ± 3.49 19 93.32 ± 5.10‡ 13 73.54 ± 10.13 Basic ADL 29 22.00 ±1.20 17 22.00 ± 1.20 18 21.50 ± 1.20 Instrumental ADL 29 53.62 ± 1.82 17 52.82 ± 1.91 18 42.00 ± 6.42§ ADCS-ADL Total 29 75.52 ± 1.82 17 74.82 ± 1.91 18 63.50 ± 7.11§ M. Marsico et al. 175 Continued EMQ Speech* 31 11.35 ± 6.19 20 19.30 ± 9.50‡ 19 36.26 ± 7.03§ EMQ Read Write* 31 3.10 ± 2.02 20 4.95 ± 3.50 19 7.05 ± 3.76 EMQ Actions* 31 3.48 ± 3.08 20 7.20 ± 4.41‡ 19 12.47 ± 3.96§ EMQ Learn New* 31 5.94 ± 2.66 20 9.05 ± 2.50‡ 19 10.68 ± 5.98 EMP Faces Places* 31 3.23 ± 2.04 20 6.60 ± 4.43‡ 19 9.47 ± 4.01§ EMQ Total* 31 27.10 ± 13.27 20 47.10 ± 22.30‡ 19 75.95 ± 18.26§ Abbreviations: HE: Healthy Elderly; aMCI: Amnestic Mild Cognitive Impairment; AD: Alzheimer’s Disease; 5cRT: Five-Choice Reaction Time; sRT: Simple Reaction Time; PALTOTEA: Paired Associates Learning Total Errors Adjusted; SWMBE: Spatial Working Memory Between Errors; ADAS- Cog: Alzheimer’s Disease Assessment Scale-Cognitive Subscale; HVLT TR: Hopkins Verbal Learning Test Total Recall; HVLT DR: Hopkins Verbal Learning Test Delayed Recall; TPT: The Placing Test; GNT: Graded Naming Test; SDMT: Symbol Digit Modalities Test; CLOX1: Clock Drawing Task 1; LCS: Letter Comparison Speed; PCS: Pattern Comparison Speed; CAMCOG: Cambridge Examination for Mental Disorders of the Elderly-Cognitive Examination; ADL: Activities of Daily Living; ADCS-ADL: Alzheimer’s Disease Cooperative Study-Activities of Daily Living; EMQ: Everyday Memory Questionnaire. * Higher score equals greater impairment. † ADAS-Cog normative data was obtained from previous literature. ‡ p < 0.05 aMCI compared to HE; significance not assessed for ADAS-Cog. § p< 0.05 AD compared to aMCI. Figure 1. Cognitive and functional z-scores for subjects with mild-to-moderate AD and aMCI normalized to HE. * Actual zscore is greater than −8. † See Table 2 for information on cognitive and functional assessments, including abbreviations. main refer to the minimum and maximum scores for the subset of measures classified within each domain. Classifications can be found in Table 2; z-scores are presented in Figure 1. Deficits were observed among AD subjects relative to aMCI subjects on each cognitive endpoint assessed, with the exception of CANTAB Spatial Working Memory. The greatest cognitive deficits observed in the AD group were within the domains of episodic memory (z-score −3.3 to −11.4), executive function (z-score -0.4 to −10.1), orientation (z-score −19.5), and semantic memory (z-score −2.4 to −10.1). Smaller deficits were observed within the domains of processing speed (z-score −0.8 to −1.8) and attention and processing (z-score −0.7 to −2.1). Measureable cognitive deficits were also observed for most endpoints among aMCI with a pattern similar to that seen in the AD group, with the largest deficits in executive function (z-score −0.1 to −4.4) and semantic memory (z-score −0.8 to −4.8) domains, and the smallest deficits in the processing speed domain (zscores > −0.2). Endpoints such as the CAMCOG and ADAS-Cog, which utilize a single score to summarize cognitive performance across a variety of domains (categorized in the tables as “general cognition”), detected large differences from HE in both AD and aMCI and were able to clearly differentiate between aMCI (CAMCOG: z-score −1.9; ADAS-Cog: z-score −1.8) and AD (CAMCOG: z-score −7.6; ADAS-Cog: z-score −5.8). Both AD and aMCI had marked deficits in executive function (Clock Drawing Task 1 (CLOX1) [42]; z-scores −10.1 and M. Marsico et al. 176 −4.4) and semantic memory (ADAS-Cog Object naming; z-scores −10.1 and −4.8). Deficits in verbal episodic memory (ADAS-Cog Recall and ADAS-Cog Recognition) were approximately twice as large in AD (z-scores −3.3 and −2.8) as compared with aMCI (z-scores −1.5 and −1.1), as were those for visuospatial memory (CANTAB PAL; AD z-score −3.4; aMCI z-score −1.5), working memory (CAMCOG Calculation; AD z-score −2.4; aMCI z-score −1.0), semantic memory (CAMCOG Comprehension; AD z-score −2.5; aMCI z- score −0.8 and CAMCOG Expression; AD z-score −2.4; aMCI z-score −1.3), and executive function (Symbol Digit Modalities Test (SDMT) [43]; AD z-score −1.7; aMCI z-score −1.0). Both AD and aMCI had deficits compared to HE in CANTAB Reaction Time tasks (AD z-score −1.9 to −2.1; aMCI z-score −1.4 to −1.6), MAP Search (AD z-score −1.6; aMCI z-score −0.6) [44], CANTAB Spatial Working Memory (AD z-score −2.1; aMCI z-score −2.4) and CAMCOG Praxis (AD z-score −2.2; aMCI z-score −1.6). Figure 2 displays mean composite z-scores by cognitive domain for domains that were assessed by at least 2 tests. 3.3. Functional Status All means and standard deviations for functional measures in the AD, aMCI, and HE groups can be found in Table 4. Figure 1 also presents the cross-sectional baseline group z-scores for the AD and aMCI groups for each of the composite and individual functional endpoints evaluated. The ADCS-ADL and EMQ total scores summarize functional status across several domains using a composite score. Measurable deficits relative to HE were observed in both AD (ADCS-ADL: z-score −6.6; EMQ: z-score −3.7) and aMCI (ADCS-ADL: z-score −0.4; EMQ: z-score −1.5). Domain-specific functional deficits were also detected; the deficits were greater among AD (z-score −0.4 to −6.4) compared to aMCI (z-score 0.0 to −1.7). There were no deficits observed in ADCS-ADL Basic ADLs for aMCI compared to HE, but impairment was seen in the ADCS-ADL’s instrumental items. The mean instrumental ADL deficit in AD (z-score −6.4) was 16-fold greater than in aMCI (z-score −0.4). Both AD and aMCI had large deficits compared to HE in EMQ sub-domains (AD z-score −1.8 to −4.0; aMCI z-score −0.9 to −1.7). Functional deficits on the EMQ Total Score were more than twice as large in AD (mean z-score −3.7) compared to aMCI (mean z-score −1.5). 4. Discussion Although both the cognitive and functional deficits in mild-to-moderate AD have been well-documented in the literature, the deficits have rarely been so well characterized and in a single cohort of participants that spans the spectrum of cognitively intact HE, aMCI and mild AD. The cognitive and functional profiles presented in this study provide insight into the comprehensive impairment among aMCI and mild AD. Detailed characterization of domain-specific cognitive and functional profiles in aMCI and mild-to-moderate AD populations plays an important role in identifying domains whose exploration may improve the sensitivity of detecting therapeutic effects in clinical trials; albeit, identification of domain-specific measures for use in Figure 2. Mean composite z-scores by domain, aMCI and mild-to-moderate AD subjects. * z-scores represent a composite of all the study’s tests that assessed the domain listed. M. Marsico et al. 177 clinical trials requires further evaluation of the psychometric properties of the tests (i.e. reliability, construct and known-groups’ validity, sensitivity to detect longitudinal change). A better understanding of expected cognitive and functional deficits across the spectrum of AD can also aid in improving the accuracy of diagnosing early and late stages of disease and differentiating between dementia subtypes [8] [21] [24]-[27]. Measurable cognitive and functional deficits were observed in aMCI compared to HE. Despite some overlap in the distribution of individual subject scores, on average, aMCI subjects performed 1 - 2 standard deviations below HE on cognitive tests and within 1 standard deviation on functional tests. The aMCI deficits were markedly less than those observed in AD. Although only a proportion of the aMCI subjects are expected to progress to AD, the consistency of their deficits suggests widespread impairment in cognition and function is present years before a clinical diagnosis of AD. Our data corroborates previous research suggesting that the most precipitous domain-specific decline on the continuum from MCI to AD conversion usually takes place in episodic memory [7]-[9]. The cognitive deficits observed in the mild-to-moderate AD group are consistent with previous research demonstrating the greatest differences from HE present in the domains of visuospatial memory (z-score −3.4 to −6.3), episodic memory (z-score −3.3 to −11.4), semantic memory (z-score −2.4 to −10.0) and executive function (z-score −0.4 to −10.1) [7]-[9] [21] [41]. The consistency of the deficits in attention and information processing in aMCI and AD suggest that deficits in higher-level cognitive processes (e.g. memory, executive function) may be associated with a fundamental attention deficit that manifests very early in the disease. The data presented here support prior experience indicating that domains other than episodic memory are compromised in aMCI or preclinical AD [45]-[47]. Based on the data presented, ADAS-Cog Immediate Recall and Recognition items, the Hopkins Verbal Learning Test revised (HVLT) Immediate and Delayed Recall [48], CAMCOG Calculation, Comprehension and Expression sub-scores, CANTAB PAL Total Adjusted Errors and the SDMT show the most promise as trial endpoints as they exhibit the ability to differentiate between HE, aMCI and AD and are appropriately scaled for these populations. General cognition scores such as the CAMCOG and ADAS-Cog total scores would appear to be less useful clinical trial endpoints since changes in total scores are more difficult to map to the underlying domain-specific impairment. Furthermore, most of the ADAS-Cog items are inappropriately scaled for use in mild AD and therefore lack the sensitivity to measure widespread domain specific impairment. As a result, in mild AD, the ADAS-Cog total score is an endpoint primarily impacted by only a couple of verbal episodic memory items (namely Word Recall and Recognition). Consequences of excluding impacted domains in a global cognition endpoint include underestimating overall impairment and the difference between mild and moderate AD and missing clinical change in mild patients when it occurs [49]. Functional decline was previously thought to be a feature that distinguished those with AD from those with more mild impairment, such that early criteria for MCI indicated that one should demonstrate “normal activities of daily living” [50]. However, as with cognitive criteria, recent revisions have noted that there may be subtle but detectable changes in functional ability in MCI as well [51]. Our data indicate a subtle but consistent impairment of functional activities in aMCI, particularly in the EMQ functional activities that correlate highly with memory performance. Activities that require use of high-level cognitive skill, including reasoning, planning, organization, and initiation abilities are more likely to be impacted in aMCI [13], perhaps because these kinds of activity are less routine and well-learned [16]. In the AD group, the instrumental ADCS-ADL and EMQ Speech domain impairment indicated is consistent with cognitive dysfunction in executive, orientation and episodic memory domains. Executive dysfunction has previously been associated with Instrumental ADL impairments [52]. A number of limitations should be considered when interpreting these results. Impairment observed in this study may not be representative of a larger and more culturally diverse sample of subjects with aMCI and mildto-moderate AD. Recruitment of a convenient community sample of well-educated Caucasians and the study’s small sample size may limit the generalizability of our findings to demographically and clinically similar populations. A major strength of the study is that it was conducted at a single study site with highly experienced neuropsychologists and nurses trained in cognitive assessments. It should however be acknowledged that the use of a single site with very qualified test administrators, which ensures high data quality and minimal loss to follow up, likely resulted in less performance-related variability than what might be observed in a multi-site, multicountry clinical trial setting. Standard scores normalized to a healthy elderly population were used to allow for simple comparison of cog- M. Marsico et al. 178 nitive and functional performance across domains. It should, however, be noted that some z-scores were generated using non-normally distributed normative data. On a number of the tests the confluence of a compressed range of performance and ceiling effects (i.e. a meaningful number of subjects scoring at the upper limit of a scale’s range) among healthy elderly resulted in z-scores among mild-to-moderate AD that may not accurately reflect the true percentage of scores that fall below a given z-score value. A cross-sectional analysis limits the ability to assess key psychometric properties of the tests, such as test-retest reliability, the presence and persistence of learning effects and the sensitivity to change due to cognitive worsening. Data on these properties is necessary to determine the appropriateness of the endpoints for clinical trials. Future analyses of these data will include comprehensive psychometric analysis of each of the study’s tests including their longitudinal performance in each of the study’s cohorts. 5. Conclusion The results of this study provide further support for comprehensive assessment and monitoring of cognitive and functional domain scores in the diagnosis and treatment of aMCI and mild-to-moderate AD. Establishing cognitive and functional profiles and assessing their change over time may inform differential diagnosis, particularly as cognitive decline is expected to be reflected in, or correlated with functional decline [53]. Domain-specific cognitive scores may be more useful than composite total scores in identifying impairment and decline in cognitive function related to regional brain function. Measuring domains such as attention, processing speed and executive function may increase the sensitivity of detecting disease progression and therapeutic effects, particularly in mild-to-moderate AD patients whose memory decline may be too slow to detect drug effects during a typical clinical trial. Further exploration of patterns of impairment may also have potential to be predictive of those at greatest risk of disease progression or who may benefit most from treatment. References [1] Levey, A., Lah, J., Goldstein, F., Steenland, K. and Bliwise, D. (2006) Mild Cognitive Impairment: An Opportunity to Identify Patients at High Risk for Progression to Alzheimer’s Disease. Clinical Therapeutics, 28, 991-1001. http://dx.doi.org/10.1016/j.clinthera.2006.07.006 [2] Petersen, R.C., Doody, R., Kurz, A., Mohs, R.C., Morris, J.C., Rabins, P.V., et al. (2001) Current Concepts in Mild Cognitive Impairment. JAMA Neurology, 58, 1985-1992. http://dx.doi.org/10.1001/archneur.58.12.1985 [3] Artero, S., Petersen, R., Touchon, J. and Ritchie, K. (2006) Revised Criteria for Mild Cognitive Impairment: Validation within a Longitudinal Population Study. Dementia and Geriatric Cognitive Disorders, 22, 465-470. http://dx.doi.org/10.1159/000096287 [4] Dubois, B., Feldman, H.H., Jacova, C., Dekosky, S.T., Barberger-Gateau, P., Cummings, J., et al. (2007) Research Criteria for the Diagnosis of Alzheimer’s Disease: Revising the NINCDS-ADRDA Criteria. Lancet Neurology, 6, 734- 746. [5] Birks, J. (2006) Cholinesterase Inhibitors for Alzheimer’s Disease. The Cochrane Database of Systematic Reviews, Article ID: CD005593. [6] Markwick, A., Zamboni, G. and de Jager, C.A. (2012) Profiles of Cognitive Subtest Impairment in the Montreal Cognitive Assessment (MoCA) in a Research Cohort with Normal Mini-Mental State Examination (MMSE) Scores. Journal of Clinical and Experimental Neuropsychology, 34, 750-757. http://dx.doi.org/10.1080/13803395.2012.672966 [7] Bondi, M.W., Jak, A.J., Delano-Wood, L., Jacobson, M.W., Delis, D.C. and Salmon, D.P. (2008) Neuropsychological Contributions to the Early Identification of Alzheimer’s Disease. Neuropsychology Review, 18, 73-90. http://dx.doi.org/10.1007/s11065-008-9054-1 [8] Karantzoulis, S. and Galvin, J.E. (2011) Distinguishing Alzheimer’s Disease from Other Major Forms of Dementia. Expert Review of Neurotherapeutics, 11, 1579-1591. http://dx.doi.org/10.1586/ern.11.155 [9] Nordlund, A., Rolstad, S., Hellstrom, P., Sjogren, M., Hansen, S. and Wallin, A. (2005) The Goteborg MCI Study: Mild Cognitive Impairment Is a Heterogeneous Condition. Journal of Neurology, Neurosurgery and Psychiatry, 76, 1485-1490. http://dx.doi.org/10.1136/jnnp.2004.050385 [10] Caine, D. and Hodges, J.R. (2001) Heterogeneity of Semantic and Visuospatial Deficits in Early Alzheimer’s Disease. Neuropsychology, 15, 155-164. http://dx.doi.org/10.1037/0894-4105.15.2.155 [11] Carter, S.F., Caine, D., Burns, A., Herholz, K. and Ralph, M.A.L. (2012) Staging of the Cognitive Decline in Alzheimer’s Disease: Insights from a Detailed Neuropsychological Investigation of Mild Cognitive Impairment and Mild Alzheimer’s Disease. International Journal of Geriatric Psychiatry, 27, 423-432. M. Marsico et al. 179 [12] Perry, R.J. and Hodges, J.R. (1999) Attention and Executive Deficits in Alzheimer’s Disease: A Critical Review. Brain, 122, 383-404. http://dx.doi.org/10.1093/brain/122.3.383 [13] Farias, S.T., Mungas, D., Reed, B.R., Harvey, D., Cahn-Weiner, D. and DeCarli, C. (2006) MCI Is Associated with Deficits in Everyday Functioning. Alzheimer Disease & Associated Disorders, 20, 217-223. http://dx.doi.org/10.1097/01.wad.0000213849.51495.d9 [14] Perneczky, R., Pohl, C., Sorg, C., Hartmann, J., Tosic, N., Grimmer, T., Heitele, S. and Kurz, A. (2006) Impairment of Activities of Daily Living Requiring Memory or Complex Reasoning as Part of the MCI Syndrome. International Journal of Geriatric Psychiatry, 21, 158-162. http://dx.doi.org/10.1002/gps.1444 [15] Reppermund, S., Brodaty, H., Crawford, J.D., Kochan, N.A., Draper, B., Slavin, M.J., et al. (2013) Impairment in Instrumental Activities of Daily Living with High Cognitive Demand Is an Early Marker of Mild Cognitive Impairment: The Sydney Memory and Ageing Study. Psychological Medicine, 43, 2437-2445. [16] Goldberg, T.E., Koppel, J., Keehlisen, L., Christen, E., Dreses-Werringloer, U., Conejero-Goldberg, C., Gordon, M.L. and Davies, P. (2010) Performance-Based Measures of Everyday Function in Mild Cognitive Impairment. American Journal of Psychiatry, 167, 845-853. http://dx.doi.org/10.1176/appi.ajp.2010.09050692 [17] Bangen, K.J., Jak, A.J., Schiehser, D.M., Delano-Wood, L., Tuminello, E., Han, S.D., et al. (2010) Complex Activities of Daily Living Vary by Mild Cognitive Impairment Subtype. Journal of the International Neuropsychological Society, 16, 630-639. http://dx.doi.org/10.1017/S1355617710000330 [18] Allaire, J.C., Gamaldo, A., Ayotte, B.J., Sims, R. and Whitfield, K. (2009) Mild Cognitive Impairment and Objective Instrumental Everyday Functioning: The Everyday Cognition Battery Memory Test. Journal of the American Geriatrics Society, 57, 120-125. http://dx.doi.org/10.1111/j.1532-5415.2008.02054.x [19] Mioshi, E., Kipps, C.M., Dawson, K., Mitchell, J., Graham, A. and Hodges, J.R. (2007) Activities of Daily Living in Frontotemporal Dementia and Alzheimer Disease. Neurology, 68, 2077-2084. http://dx.doi.org/10.1212/01.wnl.0000264897.13722.53 [20] Dangour, A.D., Allen, E., Richards, M., Whitehouse, P. and Uauy, R. (2010) Design Considerations in Long-Term Intervention Studies for the Prevention of Cognitive Decline or Dementia. Nutrition Reviews, 68, S16-S21. http://dx.doi.org/10.1111/j.1753-4887.2010.00330.x [21] de Jager, C.A., Hogervorst, E., Combrinck, M. and Budge, M.M. (2003) Sensitivity and Specificity of Neuropsychological Tests for Mild Cognitive Impairment, Vascular Cognitive Impairment and Alzheimer’s Disease. Psychological Medicine, 33, 1039-1050. http://dx.doi.org/10.1017/S0033291703008031 [22] de Jager, C.A. and Budge, M.M. (2005) Stability and Predictability of the Classification of Mild Cognitive Impairment as Assessed by Episodic Memory Test Performance over Time. Neurocase, 11, 72-79. http://dx.doi.org/10.1080/13554790490896820 [23] Hogervorst, E., Combrinck, M., Lapuerta, P., Rue, J., Swales, K. and Budge, M. (2002) The Hopkins Verbal Learning Test and Screening for Dementia. Dementia and Geriatric Cognitive Disorders, 13, 13-20. http://dx.doi.org/10.1159/000048628 [24] Kramer, J.H., Jurik, J., Sha, S.J., Rankin, K.P., Rosen, H.J., Johnson, J.K. and Miller, B.L. (2003) Distinctive Neuropsychological Patterns in Frontotemporal Dementia, Semantic Dementia, and Alzheimer Disease. Cognitive & Behavioral Neurology, 16, 211-218. http://dx.doi.org/10.1097/00146965-200312000-00002 [25] Libon, D.J., Massimo, L., Moore, P., Coslett, H.B., Chatterjee, A., Aguirre, G.K., et al. (2007) Screening for Frontotemporal Dementias and Alzheimer’s Disease with the Philadelphia Brief Assessment of Cognition: A Preliminary Analysis. Dementia and Geriatric Cognitive Disorders, 24, 441-447. http://dx.doi.org/10.1159/000110577 [26] Pendlebury, S.T., Markwick, A., de Jager, C.A., Zamboni, G., Wilcock, G.K. and Rothwell, P.M. (2012) Differences in Cognitive Profile between TIA, Stroke and Elderly Memory Research Subjects: A Comparison of the MMSE and MoCA. Cerebrovascular Diseases, 34, 48-54. http://dx.doi.org/10.1159/000338905 [27] Perry, R.J. and Hodges, J.R. (2000) Differentiating Frontal and Temporal Variant Frontotemporal Dementia from Alzheimer’s Disease. Neurology, 54, 2277-2284. http://dx.doi.org/10.1212/WNL.54.12.2277 [28] de Jager, C.A., Honey, T.E., Birks, J. and Wilcock, G.K. (2010) Retrospective Evaluation of Revised Criteria for the Diagnosis of Alzheimer’s Disease Using a Cohort with Post-Mortem Diagnosis. International Journal of Geriatric Psychiatry, 25, 988-997. http://dx.doi.org/10.1002/gps.2448 [29] Huppert, F.A., Brayne, C., Gill, C., Paykel, E.S. and Beardsall, L. (1995) CAMCOG—A Concise Neuropsychological Test to Assist Dementia Diagnosis: Socio-Demographic Determinants in an Elderly Population Sample. British Journal of Clinical Psychology, 34, 529-541. http://dx.doi.org/10.1111/j.2044-8260.1995.tb01487.x [30] Roth, M., Tym, E., Mountjoy, C.Q., Huppert, F.A., Hendrie, H., Verma, S. and Goddard, R. (1986) CAMDEX. A Standardised Instrument for the Diagnosis of Mental Disorder in the Elderly with Special Reference to the Early Detection of Dementia. British Journal of Psychiatry, 149, 698-709. http://dx.doi.org/10.1192/bjp.149.6.698 M. Marsico et al. 180 [31] Folstein, M.F., Folstein, S.E. and McHugh, P.R. (1975) “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. Journal of Psychiatric Research, 12, 189-198. http://dx.doi.org/10.1016/0022-3956(75)90026-6 [32] Brandt, J., Spencer, M. and Folstein, M. (1988) The Telephone Interview for Cognitive Status. Neuropsychiatry, Neuropsychology, & Behavioral Neurology, 1, 111-117. [33] Morris, J.C. (1997) Clinical Dementia Rating: A Reliable and Valid Diagnostic and Staging Measure for Dementia of the Alzheimer Type. International Psychogeriatrics, 9, 173-176. http://dx.doi.org/10.1017/S1041610297004870 [34] Yesavage, J.A., Brink, T.L., Rose, T.L., Lum, O., Huang, V., Adey, M. and Otto Leirer, V. (1982) Development and Validation of a Geriatric Depression Screening Scale: A Preliminary Report. Journal of Psychiatric Research, 17, 37-49. http://dx.doi.org/10.1016/0022-3956(82)90033-4 [35] Ferris, S.H., Lucca, U., Mohs, R., Dubois, B., Wesnes, K., Erzigkeit, H., et al. (1997) Objective Psychometric Tests in Clinical Trials of Dementia Drugs. Position Paper from the International Working Group on Harmonization of Dementia Drug Guidelines. Alzheimer Disease and Associated Disorders, 11, 34-38. [36] Rosen, W.G., Mohs, R.C. and Davis, K.L. (1984) A New Rating Scale for Alzheimer’s Disease. American Journal of Psychiatry, 141, 1356-1364. [37] Cambridge Cognition Ltd. (2013) Cambridge Cognition—The Home of CANTAB. CANTAB.com [Internet]. Cambridge Cognition Ltd., Cambridge, c2013. http://www.cantab.com/ [38] Galasko, D., Bennett, D., Sano, M., Ernesto, C., Thomas, R., Grundman, M. and Ferris, S. (1997) An Inventory to Assess Activities of Daily Living for Clinical Trials in Alzheimer’s Disease. Alzheimer Disease & Associated Disorders, 11, S33-S39. http://dx.doi.org/10.1097/00002093-199700112-00005 [39] Sunderland, A., Harris, J. and Baddeley, A. (1983) Do Laboratory Tests Predict Everyday Memory? A Neuropsychological Study. Journal of Verbal Learning and Verbal Behavior, 22, 341-357. http://dx.doi.org/10.1016/S0022-5371(83)90229-3 [40] Graham, D., Cully, J.A., Snow, A.L., Massman, P. and Doody, R. (2004) The Alzheimer’s Disease Assessment ScaleCognitive Subscale: Normative Data for Older Adults Controls. Alzheimer Disease and Associated Disorders, 18, 236- 240. [41] Grundman, M., Petersen, R.C., Ferris, S.H., Thomas, R.G., Aisen, P.S., Bennett, D.A., et al. (2004) Mild Cognitive Impairment Can Be Distinguished from Alzheimer Disease and Normal Aging for Clinical Trials. JAMA Neurology, 61, 59-66. http://dx.doi.org/10.1001/archneur.61.1.59 [42] Royall, D.R., Cordes, J.A. and Polk, M. (1998) CLOX: An Executive Clock Drawing Task. Journal of Neurology, Neurosurgery & Psychiatry, 64, 588-594. http://dx.doi.org/10.1136/jnnp.64.5.588 [43] Smith, A. (1968) The Symbol-Digit Modalities Test: A Neuropsychologic Test of Learning and Other Cerebral Disorders. In: Learning Disorders, Special Child Publications, Seattle, 83-91. [44] Robertson, I.H., Thames Valley Test Company (1994) The Test of Everyday Attention. Thames Valley Test Company. [45] Brandt, J., Aretouli, E., Neijstrom, M.S., Samek, J., Manning, K., Albert, M.S., et al. (2009) Selectivity of Executive Function Deficits in Mild Cognitive Impairment. Neuropsychology, 23, 607-618. http://dx.doi.org/10.1037/a0015851 [46] Papp, K.V., Snyder, P.J., Maruff, P., Bartkowiak, J. and Pietrzak, R.H. (2011) Detecting Subtle Changes in Visuospatial Executive Function and Learning in the Amnestic Variant of Mild Cognitive Impairment. PLoS ONE, 6, e21688. http://dx.doi.org/10.1371/journal.pone.0021688 [47] Voss, S. and Bullock, R. (2004) Executive Function: The Core Feature of Dementia? Dementia and Geriatric Cognitive Disorders, 18, 207-216. http://dx.doi.org/10.1159/000079202 [48] Brandt, J. (1991) The Hopkins Verbal Learning Test: Development of a New Memory Test with Six Equivalent Forms. Clinical Neuropsychologist, 5, 125-142. http://dx.doi.org/10.1080/13854049108403297 [49] Cano, S.J., Posner, H.B., Moline, M.L., Hurt, S.W., Swartz, J., Hsu, T. and Hobart, J.C. (2010) The ADAS-Cog in Alzheimer’s Disease Clinical Trials: Psychometric Evaluation of the Sum and Its Parts. Journal of Neurology, Neurosurgery & Psychiatry, 81, 1363-1368. http://dx.doi.org/10.1136/jnnp.2009.204008 [50] Petersen, R.C., Smith, G.E., Waring, S.C., Ivnik, R.J., Tangalos, E.G. and Kokmen, E. (1999) Mild Cognitive Impairment: Clinical Characterization and Outcome. JAMA Neurology, 56, 303-308. http://dx.doi.org/10.1001/archneur.56.3.303 [51] Winblad, B., Palmer, K., Kivipelto, M., Jelic, V., Fratiglioni, L., Wahlund, L.O., et al. (2004) Mild Cognitive Impairment—Beyond Controversies, towards a Consensus: Report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine, 256, 240-246. http://dx.doi.org/10.1111/j.1365-2796.2004.01380.x [52] Marshall, D.A., Rentz, D.M., Frey, M.T., Locascio, J.J., Johnson, K.A. and Sperling, R.A., the Alzheimer’s Disease Neuroimaging Initiative (2011) Executive Function and Instrumental Activities of Daily Living in Mild Cognitive Im- M. Marsico et al. 181 pairment and Alzheimer’s Disease. Alzheimer’s & Dementia, 7, 300-308. http://dx.doi.org/10.1016/j.jalz.2010.04.005 [53] Perry, R.J. and Hodges, J.R. (2000) Relationship between Functional and Neuropsychological Performance in Early Alzheimer Disease. Alzheimer Disease & Associated Disorders, 14, 1-10. http://dx.doi.org/10.1097/00002093-200001000-00001 [54] Cornish, I.M. (2000) Factor Structure of the Everyday Memory Questionnaire. British Journal of Psychology, 91, 427- 438. http://dx.doi.org/10.1348/000712600161916 [55] Salthouse, T.A. and Babcock, R.L. (1991) Decomposing Adult Age Differences in Working Memory. Developmental Psychology, 27, 763-776. http://dx.doi.org/10.1037/0012-1649.27.5.763 [56] Anderson, E., De Jager, C. and Iversen, S. (2006) The Placing Test: Preliminary Investigations of a Quick and Simple Memory Test Designed to Be Sensitive to Pre-Dementia Alzheimer’s Disease but Not to Normal Ageing. Journal of Clinical and Experimental Neuropsychology, 28, 843-858. http://dx.doi.org/10.1080/13803390591001016 [57] McKenna, P. and Warrington, E.K. (1980) Testing for Nominal Dysphasia. Journal of Neurology, Neurosurgery & Psychiatry, 43, 781-788. http://dx.doi.org/10.1136/jnnp.43.9.781
Cognitive and Functional Profiles in Mild-to-Moderate Alzheimer’s Disease and Mild Cognitive Impairment Compared to Healthy Elderly Mark Marsico1*, Celeste A. de Jager2,3, April Grant1, Xingshu Zhu4, Arwen Markwick2, Julie Chandler1 1 Epidemiology Department, Merck Research Laboratories, North Wales, USA 2 OPTIMA, Nuffield Department of Medicine, University of Oxford, Oxford, UK 3 Division of Geriatric Medicine, Department of Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa 4 Statistical Programming, Merck Research Laboratories, North Wales, USA Email: * mark.marsico@merck.com Received 24 October 2014; revised 26 November 2014; accepted 9 December 2014 Academic Editor: Lei Xue, School of Life Science, Tongji University, China Copyright © 2014 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Background: Amnestic mild cognitive impairment (aMCI) and mild-to-moderate Alzheimer’s disease (AD) are clinically distinct but impact cognitive and functional ability similarly. Comprehensive assessment of cognitive and functional deficits may prove useful in informing differential diagnosis in early stages of dementia and in informing endpoint selection in therapeutic AD trials. Objective: The objective of this study was to characterize patterns of cognitive and functional impairment in aMCI and mild-to-moderate AD subjects compared to cognitively intact healthy elderly (HE). Methods: Thirty-one healthy elderly, 20 aMCI and 19 AD participants were administered a cognitive test battery that included the ADAS-Cog and functional assessments. Z-scores were calculated for all endpoints based on the HE reference group. Results: Cognitive deficits were observed in AD and aMCI participants relative to the referent group. On average, aMCI participants performed 1 - 2 standard deviations below HE on cognitive tests, and AD participants performed 2 - 3 standard deviations below HE. Domain-specific functional deficits among AD participants (zscore −0.4 to −6.4) were consistently greater than those of aMCI participants (z-score 0 to −1.7). Conclusion: This study provides further support for comprehensive assessment and monitoring of * Corresponding author. M. Marsico et al. 169 cognitive and functional domain scores in the diagnosis and treatment of aMCI and mild AD. Domain-specific cognitive scores may be more useful than composite scores in characterizing impairment and decline. Measuring domains such as attention, processing speed and executive function may increase the sensitivity of detecting disease progression and therapeutic effects, particularly in mild-moderate AD where memory decline may be too slow to detect drug effects during a typical clinical trial. Keywords Alzheimer’s Disease, Amnestic Mild Cognitive Impairment, Dementia, Cognition 1. Introduction Mild Cognitive Impairment (MCI) is characterized by changes in cognition which are less severe and widespread than those of Alzheimer’s disease (AD), but represent a decline from cognitive functioning of normal aging and predisposes one to the risk of developing AD [1]. Initially, recognition of MCI was identified by detection of memory performance below age-associated norms [2]. However, recent revisions of this syndrome acknowledge that deficits may also occur in other cognitive domains [3]. Cognitive decline is known to be preceded by neuropathology including beta-amyloid plaque deposition, neurofibrillary tangle formation and atrophy in the brain. Novel research with neuroimaging and CSF markers of pathology has led to revised research criteria for AD diagnosis [4]. The criteria include memory impairment as assessed with sensitive episodic memory tests. This new diagnostic framework has stimulated debate about the definition of AD and related conditions. The Mini-Mental State Examination (MMSE) and Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog) have typically been used as outcome measures in AD drug intervention trials [5]; however, these measures may be insensitive for detecting early and subclinical changes [6]. It is widely accepted that the typical cognitive profile in early AD is marked by episodic memory impairment with semantic memory, working memory, executive functioning, and visuospatial and attentional dysfunction emerging as the disease progresses [7]-[9]. The heterogeneity of cognitive decline suggests endpoints that comprehensively assess cognition may be best suited for detecting cognitive change [10]-[12]. AD is also marked by deficits in functional ability, characterized by progressive deterioration of activities of daily living (ADL), including early decline in instrumental activities, and later activities relating to basic selfcare [13]. Once loss of ADL occurs and independent living is problematic, diagnosis will include dementia. ADL impairments associated with MCI may occur for instrumental activities that require high-level cognitive skills [14] [15] such as use of technology, understanding cultural expectations [16], managing money [17], medication use [18] and executive functioning [13]. As with cognitive measures, indications of differential profiles of functional impairment have also been found for AD as compared to other dementia groups [19]. Countless cognitive tests have been used worldwide [20] to assess cognitive change in observational studies and clinical trials. In many instances, cognitive tests are included alongside the MMSE and ADAS-Cog in order to evaluate the potential utility of the measures as compared to these “gold standard” trial endpoints. An objective of the Oxford Project to Investigate Memory and Aging (OPTIMA) study was to identify cognitive tests capable of discriminating between cognitively healthy and cognitively impaired groups [21]-[23] as a first step in identifying the tests most likely to identify preclinical AD and to aid in diagnosis of dementia types. For this study, an adjunct to the original OPTIMA cohort, a selection of cognitive measures was added to the OPTIMA battery with the objective of identifying psychometrically sound cognitive measures for use as cognition endpoints in future AD clinical trials. The purpose of this analysis was to determine, in a single cohort of participants, the pattern of cognitive and functional impairment of amnestic MCI-subtype (aMCI) and AD participants, using scores from HE as a reference. Clarification of cognitive and functional profiles is important for accurate differential diagnosis and monitoring decline across dementia syndromes [8] [24]-[27]. Furthermore, comprehensive understanding of domainspecific cognitive and functional profiles in aMCI and AD is the first step in identifying particular measures that are sensitive to impairment and the domains that can be utilized as endpoints to better characterize disease and assess impact on the early treatment to mild AD in clinical trials. M. Marsico et al. 170 2. Materials and Methods 2.1. Participants Participants were recruited from a larger cohort of living subjects enrolled in the Oxford Project to Investigate Memory and Aging (OPTIMA) study within the United Kingdom [28], a longitudinal study of memory and aging established at the University of Oxford. All enrolled subjects undergo a clinical and informant-based interview, a comprehensive cognitive assessment including the Cambridge Cognitive Examination (CAMCOG) [29] [30] and ADAS-Cog, physical examination, MRI brain scan and blood-screening tests as part of the scheduled protocol for diagnostic purposes. Potential participants for the current prospective study were identified from the OPTIMA participant database according to clinical diagnosis at last visit. Those with Mini-Mental State Examination (MMSE) [31] score of ≥16 (N = 224) had their medical records screened for inclusion/exclusion criteria and rescreened to assess suitability for inclusion in the study. Sixty one subjects were excluded (27%) based on a review of their medical record. A further 73 (33%) were excluded after not responding to study invitation letters and telephone calls. The most common reasons for exclusion based on the chart review and telephone screen were: acute illnesses, recent stroke, advanced cancer, other dementia diagnosis than AD, advanced dementia or unwillingness to participate. The remaining 90 subjects (40%) were screened by telephone. Current dementia medication and administration of the Telephone Interview for Cognitive Status-Modified (TICS-M) [32] were used to establish a preliminary diagnostic classification. General inclusion criteria applicable to all subjects for this sub-study, except where noted, were 60 years of age or older, no medical condition affecting cognition (excluding AD), a reliable informant willing to act as a study partner (not required for HE), adequate sensory and motor capabilities to perform cognitive testing, fluency in English as determined by the investigator; and the ability to understand study procedures and give verbal consent to participate. Exclusions included: a history of disease that might confound study results, a significant psychiatric history, recent (within 2 years) or current evidence of major untreated depressive or psychiatric disorder, uncontrolled or untreated endocrine disease or other medical condition causing transient or continuous alteration of consciousness or attention, recent (within 2 years) or current evidence of major stroke, multiple lacunar infarcts, transient ischemic events (within 3 months), epilepsy, Parkinson’s disease, progressive supranuclear palsy, Huntington’s disease, amyotrophic lateral sclerosis, multiple sclerosis or other central neurological disorder or a history of significant head trauma with loss of consciousness. Final diagnostic classification was made at a consensus meeting of at least two research staff consisting of neuropsychologists and nurses. Cognitive and functional cohort specific diagnostic classification criteria are provided in Table 1. 2.2. Procedures In addition to screening and diagnostic assessments (i.e. Clinical Dementia Rating (CDR) [33], MMSE, Subjective Memory Complaint (SMC) [30] and Geriatric Depression Scale (GDS) [34]), eligible subjects underwent cognitive testing and completed functional and self- and informant-reported assessments at the screening visit. Cognitive and functional data collected at this visit were considered “baseline” data. At subsequent study visits, administrations of cognitive and functional measures were repeated. Information about changes in subject health Table 1. Cognitive and functional diagnostic classification criteria. HE aMCI AD • CDR = 0 • MMSE ≥ 28 • GDS < 20 • TICS-M total score ≥ 29 • TICS-m WLR ≥ 11 • No significant SMC • Without evidence of depression • Without dementia of ADL impairment • CDR ≤ 0.5 • MMSE ≥ 23 • GDS < 20 • TICS-M total score < 29 and > 14 • TICS-m WLR < 10 • Without dementia and with minimal or no ADL impairment • Met Petersen criteria including the presence of SMC • CDR > 0.5 • MMSE ≥ 16 • GDS < 20 • TICS-M total score < 29 and > 14 • TICS-m WLR < 10 • Possible or probable AD according to previous or new clinical diagnosis using the NINCDS-ADRDA criteria Abbreviations: CDR: Clinical Dementia Rating; MMSE: Mini-Mental State Examination; GDS: Geriatric Depression Scale; TICS-M: Telephone Interview for Cognitive Status-Modified; WLR: Word List Recall; SMC: Subjective Memory Complaint; ADL: Activities of Daily Living; NINCDSADRDA: National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association. M. Marsico et al. 171 or medical conditions, including changes in medication use and medical events occurring between visits, was collected. The structure of study visits and sequence of study assessments were consistent for all subjects. All study procedures and assessments were administered by either trained research nurses or research neuropsychologists. Only the study’s baseline, cross-sectional data are presented in this paper. Longitudinal data collected across subsequent visits (6 and 12 months after baseline) are not presented. Subjects were not administered any marketed or investigational compound in this study. Subjects were however, undergoing usual care by their healthcare providers for their disease which may have included medication indicated for the treatment of AD. Because most subjects began their AD treatment prior to their inclusion in the study, responsiveness (i.e. the ability of a measure to detect pharmacological change) could not be assessed. All study procedures were in accord with the ethical standards of the Committee on Human Experimentation of the institution in which the experiments were done or in accord with the Helsinki Declaration of 1975. Informed consent was taken on recruitment into the OPTIMA cohort. Ethical approval for this study was granted by the Frenchay Regional Ethics Committee, reference 09/H0107/09. 2.3. Measures Cognitive tests were chosen for this study based on published evidence of the tests’ psychometric properties and their usefulness in assessing cognition in AD and aMCI [35]. Tests sensitive to mild impairment were chosen for comparison with the broadly used ADAS-Cog. The overarching aim in selecting cognitive measures was to achieve broad domain coverage with at least two measures assessing each cognitive domain known to be impacted in AD. The cognitive test battery included the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADASCog) [36]; the Cambridge Automated Neuropsychological Test Battery (CANTAB) [37] Paired Associate Learning (PAL), Reaction Time Index (RTI) and Spatial Working Memory tests; the CAMCOG; and domainspecific tests in verbal and visuospatial episodic memory, semantic memory, attention, executive function and information processing speed (see Table 2). Functional measures included the Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL) [38], scored on basic and instrumental ADL, and the Everyday Memory Questionnaire (EMQ) [39]. Detailed description of all study measures can be found in the online supplement. 2.4. Statistical Methods Standardized z-scores were calculated for all cognitive and functional baseline data based on the mean and standard deviation scores in the HE group. Standardizing the aMCI and mild-to-moderate AD performance to a z-score based on a HE sample allows all test and domain scores to be reported based on the same scale with mean of 0 and a standard deviation of 1. Domain composite scores were calculated by summing the individual tests’ z-score means and dividing by the number of tests comprising that domain. Individual cognitive tests were grouped according to domain as indicated in Table 2. The effects of age and education were assessed using one-way analysis of variance (ANOVA); independent t-tests were conducted to investigate the potential influence of gender. A p-value of <0.05 was considered significant for all comparisons. Because cognitive performance was not impacted by any of these covariates (data not shown), unadjusted, raw data were used in all z-score calculations. Because the ADAS-Cog was not administered to HE in this study, standard scores were created using previously published normative data [40] [41]. This study was designed to descriptively assess cognitive performance and functional status in non-demented, aMCI and mild-to-moderate AD. The study was not powered to support formal statistical testing comparing the cognitive and functional outcomes across the three groups. However, statistical tests (ANOVA) were performed and p-values presented to facilitate interpretation of the differences between groups with regard to their cognitive and functional scores. Imputation was not performed for missing data so the number of subjects contributing to group means may vary by endpoint. All statistical analyses were performed using SAS version 9.3. 3. Results 3.1. Demographic Characteristics Seventy five OPTIMA subjects prescreened with the TICS-M were invited to come into the study clinic for a M. Marsico et al. 172 Table 2. Cognitive and functional test characteristics. Cognitive/ Functional Battery Test Name Abbreviation Cognitive Domain Outcome Variables Range CANTAB Computerized Battery† CANTAB Reaction Time 5-Choice Movement Time* 5cRT Movement Attention & Processing Milliseconds 0 - ∞ CANTAB Reaction Time 5-Choice Reaction Time* 5cRT Reaction Attention & Processing Milliseconds 0 - ∞ CANTAB Reaction Time Simple Movement Time* sRT Movement Attention & Processing Milliseconds 0 - ∞ CANTAB Reaction Time Simple Reaction Time* sRT Reaction Attention & Processing Milliseconds 0 - ∞ CANTAB Paired Associates Learning Total Errors* PALTOTEA Visuospatial Memory Total Errors Adjusted 0 - 158 CANTAB Spatial Working Memory Between Errors* SWMBE Working Memory Errors 0 - 326 ADAS-Cog and Subtests‡,§ ADAS-Cog Total Score* ADAS-Cog Total General Cognition Total Errors 0 - 70 ADAS-Cog Naming Objects and Fingers* ADAS-cog Objects Semantic Memory Total Incorrect 0 - 17 ADAS-Cog Word Recall* ADAS-cog Recall Verbal Episodic Memory Mean Words per Trial Not Recalled 0 - 10 ADAS-Cog Word Recognition* ADAS-Cog Recognition Verbal Episodic Memory Words Not Recognized 0 - 24 Mazes Seconds* Mazes Executive Function Seconds 0 - 240 OPTIMA‡ Hopkins Verbal Learning Test (Total Recall) HVLT TR Verbal Episodic Memory Words Recalled 0 - 36 Hopkins Verbal Learning Test (Delayed Recall) HVLT DR Verbal Episodic Memory Words Recalled 0 - 12 The Placing Test TPT Objects Visuospatial Memory Number Correct 0 - 10 CAMCOG Learning Learn Episodic Memory Number Correct 0 - 17 CAMCOG Recent Memory Recent Episodic Memory Number Correct 0 - 4 CAMCOG Remote Memory Remote Episodic Memory Number Correct 0 - 6 CAMCOG Comprehension Comprehension Semantic Memory Summed Ordinal Scale 0 - 9 CAMCOG Expression Expression Semantic Memory Summed Ordinal Scale 0 - 21 Graded Naming Test GNT Semantic Memory Number Correct 0 - 30 CAMCOG Calculation Calculation Working Memory Number Correct 0 - 2 Symbol Digit Modalities Test SDMT Executive Function Completed Minus Errors 0 - 110 Clock Drawing Task 1 CLOX 1 Executive Function Number Correct 0 - 15 CAMCOG Abstract Thinking Thinking Executive Function Summed Ordinal Scale 0 - 8 CAMCOG Orientation Orientation Orientation Number Correct 0 - 10 CAMCOG Perception Perception Perception Number Correct 0 - 11 CAMCOG Praxis Praxis Praxis Number Correct 0 - 12 CAMCOG Attention Attention Attention & Processing Accuracy & Summed Scale 0 - 7 Map Search from the Tests for Everyday Attention MAP Search Attention & Processing Items Circled 0 - 80 Letter Comparison Speed LCS Processing Speed Accuracy Score 0 - 20 Pattern Comparison Speed PCS Processing Speed Accuracy Score 0 - 30 Cambridge Cognitive Examination (CAMCOG total score) CAMCOG Total General Cognition Subscales Total Score 0 - 107 ADCS-ADL¶ ADCS-ADL Basic Activities of Daily Living Basic ADL Functioning Summed Item Score 0 - 18 ADCS-ADL Instrumental Activities of Daily Living Instrumental ADL Functioning Summed Item Score 0 - 60 M. Marsico et al. 173 Continued ADCS-ADL Total Score ADCS-ADL Total Functioning Summed Item Score 0 - 78 Everyday Memory Questionnaire¶ EMQ Speech* EMQ Speech Functioning Summed Item Score 0 - 52 EMQ Reading and Writing* EMQ Read Write Functioning Summed Item Score 0 - 16 EMQ Actions* EMQ Actions Functioning Summed Item Score 0 - 24 EMQ Learning New Things* EMQ Learn New Functioning Summed Item Score 0 - 24 EMQ Faces and Places* EMQ Faces Places Functioning Summed Item Score 0 - 24 EMQ Total Score* EMQ Total Functioning Summed Item Score 0 - 140 Abbreviations: CANTAB: Cambridge Neuropsychological Test Automated Battery [37]; ADAS-Cog: Alzheimer’s Disease Assessment Scale-Cognitive Subscale; OPTIMA: Oxford Project to Investigate Memory and Aging; CAMCOG: Cambridge Cognitive Examination; ADCS-ADL: Alzheimer’s Disease Cooperative Study-Activities of Daily Living; EMQ: Everyday Memory Questionnaire. * Higher score equals greater impairment. † Mode of administration is via a Central Processing Unit (CPU). ‡ Mode of administration is paper and pencil. § ADAS-Cog normative data was obtained from previous literature. ¶ Mode of administration is interview-based. Table 3. Baseline demographic characteristics and cognitive function scores. Baseline Characteristics Healthy Elderly (n = 31) Amnestic Mild Cognitive Impairment (n = 20) Alzheimer’s Disease (n = 19) p Value* Age, mean years (SD) 80.94 (5.47) 80.85 (5.38) 77.79 (6.90) .15 Gender, males (%) 13 (41.94) 16 (80.00) 11 (57.89) .03 Education, mean years (SD) 14 (2.92) 13 (2.84) 13 (3.00) .63 MMSE, mean score (SD)† 29.52 (0.68) 27.80 (1.20) 22.89 (2.87 ) <.001 GDS, mean score (SD)‡ 4.10 (3.92) 6.40 (3.98) 7.50 (4.20) .01 Baseline ADCS-ADL Instrumental, mean score (SD)§ 53.52 (1.82) 52.82 (1.91) 42.00 (6.42) <.001 Baseline EMQ, mean score (SD)¶ 27.10 (13.27) 47.10 (22.30) 75.95 (18.26) <.001 Abbreviations: MMSE: Mini-Mental State Examination; GDS: Geriatric Depression Scale; ADCS-ADL: Alzheimer’s Disease Cooperative Study-Activities of Daily Living; EMQ: Everyday Memory Questionnaire. * ANOVA was used to calculate p values for continuous variables and chi-square test for categorical variables. † MMSE scores range from 0 to 30, with higher scores indicating less impairment. ‡ GDS scores range from 0 to 30, with higher scores indicating more depressive symptoms. § ADCS-ADL Instrumental scores range from 0 to 60, with higher scores indicating less impairment. ¶ EMQ scores range from 0 to 140, with higher scores indicating greater dysfunction. baseline visit. Only 5 (7%) participants (all MCI or AD) failed to meet inclusion criteria. The final sample consisted of 31 HE, 20 aMCI and 19 AD. Results comparing demographic factors by group are presented in Table 3. On average, AD subjects had less education than the HE and were approximately 3 years younger than the HE and aMCI, but the differences were not statistically significant. There were significantly more males recruited into the aMCI and AD groups compared to HE. MMSE scores were statistically different across the groups with highest scores among the HE (29.5 ± 0.7) and aMCI (27.8 ± 1.2) compared to AD (22.9 ± 2.9). A gradient of increasing functional impairment and depressive symptoms with increasing cognitive impairment was observed between the groups. At baseline, 8 (42%) mild-to-moderate AD subjects had received cognitive-enhancing treatment as part of their usual medical care for an average of 3.9 ± 3.2 years. 3.2. Cognitive Performance All means and standard deviations for cognitive test performance in the mild-to-moderate AD, aMCI, and HE groups can be found in Table 4. Figure 1 presents the cross-sectional baseline z-scores for AD and aMCI, grouped by cognitive domain, for each of the cognitive endpoints evaluated. Cut-off lines of 1.5 and 2 standard deviations below the mean are indicated as the usual thresholds of significant deficit from normal control performance for MCI and AD, respectively [2]. All references made to the deficits observed in AD and aMCI groups are always relative to the HE referent group. Minimum and maximum z-scores within a cognitive do- M. Marsico et al. 174 Table 4. Means and standard deviations for cognitive and functional tests. Test Abbreviation N HE Mean ± SD N aMCI Mean ± SD N AD Mean ± SD 5cRT Movement* 31 311.32 ± 100.74 20 335.75 ± 117.27 17 425.76 ± 171.22 5cRT Reaction* 31 383.94 ± 51.94 20 466.10 ± 73.63‡ 17 491.71 ± 112.49 sRT Movement* 31 312.26 ± 158.18 20 311.75 ± 131.15 17 444.00 ± 213.33 sRT Reaction* 31 342.65 ± 65.17 20 431.70 ± 114.87 17 466.12 ± 254.93 PALTOTEA* 30 37.80 ± 23.93 19 74.53 ± 33.27‡ 18 117.94 ± 18.53 SWMBE* 31 62.84 ± 15.76 20 25.60 ± 13.00‡ 17 30.35 ± 5.59 ADAS-Cog Total* 107 5.6 ± 3.3† 20 11.38 ± 4.78 19 24.74 ± 10.40§ ADAS-Cog Objects* 124 0.05 ± 0.22† 20 1.10 ± 0.97 19 2.26 ± 2.21§ ADAS-Cog Recall* 107 2.7 ± 1.2† 20 4.53 ± 1.30 19 6.63 ± 1.60§ ADAS-Cog Recognition* 107 2.0 ± 2.2† 20 4.35 ± 3.15 19 8.16 ± 3.85§ Mazes* 107 30.0 ± 27.9† 20 33.00 ± 15.50 19 40.21 ± 16.51 HVLT TR 31 24.35 ± 4.32 19 19.05 ± 4.56‡ 19 11.32 ± 5.20§ HVLT DR 30 8.13 ± 2.29 19 5.79 ± 2.42‡ 19 0.05 ± 0.23§ TPT Objects 31 9.29 ± 0.90 19 8.58 ± 1.98 19 3.58 ± 1.87§ Learn 31 13.74 ± 1.79 19 11.26 ± 2.51‡ 13 4.38 ± 1.45§ Recent 31 3.97 ± 0.18 19 3.79 ± 0.54 13 1.92 ± 1.04§ Remote 31 5.45 ± 0.62 19 5.11 ± 0.88 13 3.38 ± 0.96§ Comprehension 31 8.94 ± 0.25 19 8.74 ± 0.56 13 8.31 ± 0.63§ Expression 31 19.52 ± 1.31 19 17.82 ± 1.07‡ 13 16.38 ± 2.87 GNT 31 26.35 ± 3.22 19 23.26 ± 4.17 19 14.05 ± 8.39§ Calculation 31 1.97 ± 0.18 19 1.79 ± 0.54 13 1.54 ± 0.66 SDMT 12 40.33 ± 9.59 11 30.91 ± 10.63 18 24.06 ± 11.83 CLOX 1 5 14.20 ± 0.45 9 12.22 ± 1.72 12 9.67 ± 3.87 Thinking 31 7.68 ± 0.60 19 7.16 ± 1.01 13 5.85 ± 2.12§ Orientation 31 9.97 ± 0.18 19 9.53 ± 0.70 13 6.46 ± 1.85§ Perception 31 10.42 ± 0.85 19 10.32 ± 1.16 13 8.54 ± 2.03§ Praxis 31 11.68 ± 0.48 19 10.89 ± 1.15‡ 13 10.62 ± 1.12 Attention 31 6.68 ± 0.79 19 6.89 ± 0.32 13 6.15 ± 1.07§ MAP Search 31 52.29 ± 13.86 18 43.72 ± 14.93 19 30.05 ± 16.02§ LCS 10 7.70 ± 2.50 10 7.30 ± 2.06 15 5.60 ± 2.13 PCS 10 11.60 ± 1.51 9 11.78 ± 2.59 15 8.93 ± 2.31§ CAMCOG Total 31 100.00 ± 3.49 19 93.32 ± 5.10‡ 13 73.54 ± 10.13 Basic ADL 29 22.00 ±1.20 17 22.00 ± 1.20 18 21.50 ± 1.20 Instrumental ADL 29 53.62 ± 1.82 17 52.82 ± 1.91 18 42.00 ± 6.42§ ADCS-ADL Total 29 75.52 ± 1.82 17 74.82 ± 1.91 18 63.50 ± 7.11§ M. Marsico et al. 175 Continued EMQ Speech* 31 11.35 ± 6.19 20 19.30 ± 9.50‡ 19 36.26 ± 7.03§ EMQ Read Write* 31 3.10 ± 2.02 20 4.95 ± 3.50 19 7.05 ± 3.76 EMQ Actions* 31 3.48 ± 3.08 20 7.20 ± 4.41‡ 19 12.47 ± 3.96§ EMQ Learn New* 31 5.94 ± 2.66 20 9.05 ± 2.50‡ 19 10.68 ± 5.98 EMP Faces Places* 31 3.23 ± 2.04 20 6.60 ± 4.43‡ 19 9.47 ± 4.01§ EMQ Total* 31 27.10 ± 13.27 20 47.10 ± 22.30‡ 19 75.95 ± 18.26§ Abbreviations: HE: Healthy Elderly; aMCI: Amnestic Mild Cognitive Impairment; AD: Alzheimer’s Disease; 5cRT: Five-Choice Reaction Time; sRT: Simple Reaction Time; PALTOTEA: Paired Associates Learning Total Errors Adjusted; SWMBE: Spatial Working Memory Between Errors; ADAS- Cog: Alzheimer’s Disease Assessment Scale-Cognitive Subscale; HVLT TR: Hopkins Verbal Learning Test Total Recall; HVLT DR: Hopkins Verbal Learning Test Delayed Recall; TPT: The Placing Test; GNT: Graded Naming Test; SDMT: Symbol Digit Modalities Test; CLOX1: Clock Drawing Task 1; LCS: Letter Comparison Speed; PCS: Pattern Comparison Speed; CAMCOG: Cambridge Examination for Mental Disorders of the Elderly-Cognitive Examination; ADL: Activities of Daily Living; ADCS-ADL: Alzheimer’s Disease Cooperative Study-Activities of Daily Living; EMQ: Everyday Memory Questionnaire. * Higher score equals greater impairment. † ADAS-Cog normative data was obtained from previous literature. ‡ p < 0.05 aMCI compared to HE; significance not assessed for ADAS-Cog. § p< 0.05 AD compared to aMCI. Figure 1. Cognitive and functional z-scores for subjects with mild-to-moderate AD and aMCI normalized to HE. * Actual zscore is greater than −8. † See Table 2 for information on cognitive and functional assessments, including abbreviations. main refer to the minimum and maximum scores for the subset of measures classified within each domain. Classifications can be found in Table 2; z-scores are presented in Figure 1. Deficits were observed among AD subjects relative to aMCI subjects on each cognitive endpoint assessed, with the exception of CANTAB Spatial Working Memory. The greatest cognitive deficits observed in the AD group were within the domains of episodic memory (z-score −3.3 to −11.4), executive function (z-score -0.4 to −10.1), orientation (z-score −19.5), and semantic memory (z-score −2.4 to −10.1). Smaller deficits were observed within the domains of processing speed (z-score −0.8 to −1.8) and attention and processing (z-score −0.7 to −2.1). Measureable cognitive deficits were also observed for most endpoints among aMCI with a pattern similar to that seen in the AD group, with the largest deficits in executive function (z-score −0.1 to −4.4) and semantic memory (z-score −0.8 to −4.8) domains, and the smallest deficits in the processing speed domain (zscores > −0.2). Endpoints such as the CAMCOG and ADAS-Cog, which utilize a single score to summarize cognitive performance across a variety of domains (categorized in the tables as “general cognition”), detected large differences from HE in both AD and aMCI and were able to clearly differentiate between aMCI (CAMCOG: z-score −1.9; ADAS-Cog: z-score −1.8) and AD (CAMCOG: z-score −7.6; ADAS-Cog: z-score −5.8). Both AD and aMCI had marked deficits in executive function (Clock Drawing Task 1 (CLOX1) [42]; z-scores −10.1 and M. Marsico et al. 176 −4.4) and semantic memory (ADAS-Cog Object naming; z-scores −10.1 and −4.8). Deficits in verbal episodic memory (ADAS-Cog Recall and ADAS-Cog Recognition) were approximately twice as large in AD (z-scores −3.3 and −2.8) as compared with aMCI (z-scores −1.5 and −1.1), as were those for visuospatial memory (CANTAB PAL; AD z-score −3.4; aMCI z-score −1.5), working memory (CAMCOG Calculation; AD z-score −2.4; aMCI z-score −1.0), semantic memory (CAMCOG Comprehension; AD z-score −2.5; aMCI z- score −0.8 and CAMCOG Expression; AD z-score −2.4; aMCI z-score −1.3), and executive function (Symbol Digit Modalities Test (SDMT) [43]; AD z-score −1.7; aMCI z-score −1.0). Both AD and aMCI had deficits compared to HE in CANTAB Reaction Time tasks (AD z-score −1.9 to −2.1; aMCI z-score −1.4 to −1.6), MAP Search (AD z-score −1.6; aMCI z-score −0.6) [44], CANTAB Spatial Working Memory (AD z-score −2.1; aMCI z-score −2.4) and CAMCOG Praxis (AD z-score −2.2; aMCI z-score −1.6). Figure 2 displays mean composite z-scores by cognitive domain for domains that were assessed by at least 2 tests. 3.3. Functional Status All means and standard deviations for functional measures in the AD, aMCI, and HE groups can be found in Table 4. Figure 1 also presents the cross-sectional baseline group z-scores for the AD and aMCI groups for each of the composite and individual functional endpoints evaluated. The ADCS-ADL and EMQ total scores summarize functional status across several domains using a composite score. Measurable deficits relative to HE were observed in both AD (ADCS-ADL: z-score −6.6; EMQ: z-score −3.7) and aMCI (ADCS-ADL: z-score −0.4; EMQ: z-score −1.5). Domain-specific functional deficits were also detected; the deficits were greater among AD (z-score −0.4 to −6.4) compared to aMCI (z-score 0.0 to −1.7). There were no deficits observed in ADCS-ADL Basic ADLs for aMCI compared to HE, but impairment was seen in the ADCS-ADL’s instrumental items. The mean instrumental ADL deficit in AD (z-score −6.4) was 16-fold greater than in aMCI (z-score −0.4). Both AD and aMCI had large deficits compared to HE in EMQ sub-domains (AD z-score −1.8 to −4.0; aMCI z-score −0.9 to −1.7). Functional deficits on the EMQ Total Score were more than twice as large in AD (mean z-score −3.7) compared to aMCI (mean z-score −1.5). 4. Discussion Although both the cognitive and functional deficits in mild-to-moderate AD have been well-documented in the literature, the deficits have rarely been so well characterized and in a single cohort of participants that spans the spectrum of cognitively intact HE, aMCI and mild AD. The cognitive and functional profiles presented in this study provide insight into the comprehensive impairment among aMCI and mild AD. Detailed characterization of domain-specific cognitive and functional profiles in aMCI and mild-to-moderate AD populations plays an important role in identifying domains whose exploration may improve the sensitivity of detecting therapeutic effects in clinical trials; albeit, identification of domain-specific measures for use in Figure 2. Mean composite z-scores by domain, aMCI and mild-to-moderate AD subjects. * z-scores represent a composite of all the study’s tests that assessed the domain listed. M. Marsico et al. 177 clinical trials requires further evaluation of the psychometric properties of the tests (i.e. reliability, construct and known-groups’ validity, sensitivity to detect longitudinal change). A better understanding of expected cognitive and functional deficits across the spectrum of AD can also aid in improving the accuracy of diagnosing early and late stages of disease and differentiating between dementia subtypes [8] [21] [24]-[27]. Measurable cognitive and functional deficits were observed in aMCI compared to HE. Despite some overlap in the distribution of individual subject scores, on average, aMCI subjects performed 1 - 2 standard deviations below HE on cognitive tests and within 1 standard deviation on functional tests. The aMCI deficits were markedly less than those observed in AD. Although only a proportion of the aMCI subjects are expected to progress to AD, the consistency of their deficits suggests widespread impairment in cognition and function is present years before a clinical diagnosis of AD. Our data corroborates previous research suggesting that the most precipitous domain-specific decline on the continuum from MCI to AD conversion usually takes place in episodic memory [7]-[9]. The cognitive deficits observed in the mild-to-moderate AD group are consistent with previous research demonstrating the greatest differences from HE present in the domains of visuospatial memory (z-score −3.4 to −6.3), episodic memory (z-score −3.3 to −11.4), semantic memory (z-score −2.4 to −10.0) and executive function (z-score −0.4 to −10.1) [7]-[9] [21] [41]. The consistency of the deficits in attention and information processing in aMCI and AD suggest that deficits in higher-level cognitive processes (e.g. memory, executive function) may be associated with a fundamental attention deficit that manifests very early in the disease. The data presented here support prior experience indicating that domains other than episodic memory are compromised in aMCI or preclinical AD [45]-[47]. Based on the data presented, ADAS-Cog Immediate Recall and Recognition items, the Hopkins Verbal Learning Test revised (HVLT) Immediate and Delayed Recall [48], CAMCOG Calculation, Comprehension and Expression sub-scores, CANTAB PAL Total Adjusted Errors and the SDMT show the most promise as trial endpoints as they exhibit the ability to differentiate between HE, aMCI and AD and are appropriately scaled for these populations. General cognition scores such as the CAMCOG and ADAS-Cog total scores would appear to be less useful clinical trial endpoints since changes in total scores are more difficult to map to the underlying domain-specific impairment. Furthermore, most of the ADAS-Cog items are inappropriately scaled for use in mild AD and therefore lack the sensitivity to measure widespread domain specific impairment. As a result, in mild AD, the ADAS-Cog total score is an endpoint primarily impacted by only a couple of verbal episodic memory items (namely Word Recall and Recognition). Consequences of excluding impacted domains in a global cognition endpoint include underestimating overall impairment and the difference between mild and moderate AD and missing clinical change in mild patients when it occurs [49]. Functional decline was previously thought to be a feature that distinguished those with AD from those with more mild impairment, such that early criteria for MCI indicated that one should demonstrate “normal activities of daily living” [50]. However, as with cognitive criteria, recent revisions have noted that there may be subtle but detectable changes in functional ability in MCI as well [51]. Our data indicate a subtle but consistent impairment of functional activities in aMCI, particularly in the EMQ functional activities that correlate highly with memory performance. Activities that require use of high-level cognitive skill, including reasoning, planning, organization, and initiation abilities are more likely to be impacted in aMCI [13], perhaps because these kinds of activity are less routine and well-learned [16]. In the AD group, the instrumental ADCS-ADL and EMQ Speech domain impairment indicated is consistent with cognitive dysfunction in executive, orientation and episodic memory domains. Executive dysfunction has previously been associated with Instrumental ADL impairments [52]. A number of limitations should be considered when interpreting these results. Impairment observed in this study may not be representative of a larger and more culturally diverse sample of subjects with aMCI and mildto-moderate AD. Recruitment of a convenient community sample of well-educated Caucasians and the study’s small sample size may limit the generalizability of our findings to demographically and clinically similar populations. A major strength of the study is that it was conducted at a single study site with highly experienced neuropsychologists and nurses trained in cognitive assessments. It should however be acknowledged that the use of a single site with very qualified test administrators, which ensures high data quality and minimal loss to follow up, likely resulted in less performance-related variability than what might be observed in a multi-site, multicountry clinical trial setting. Standard scores normalized to a healthy elderly population were used to allow for simple comparison of cog- M. Marsico et al. 178 nitive and functional performance across domains. It should, however, be noted that some z-scores were generated using non-normally distributed normative data. On a number of the tests the confluence of a compressed range of performance and ceiling effects (i.e. a meaningful number of subjects scoring at the upper limit of a scale’s range) among healthy elderly resulted in z-scores among mild-to-moderate AD that may not accurately reflect the true percentage of scores that fall below a given z-score value. A cross-sectional analysis limits the ability to assess key psychometric properties of the tests, such as test-retest reliability, the presence and persistence of learning effects and the sensitivity to change due to cognitive worsening. Data on these properties is necessary to determine the appropriateness of the endpoints for clinical trials. Future analyses of these data will include comprehensive psychometric analysis of each of the study’s tests including their longitudinal performance in each of the study’s cohorts. 5. Conclusion The results of this study provide further support for comprehensive assessment and monitoring of cognitive and functional domain scores in the diagnosis and treatment of aMCI and mild-to-moderate AD. Establishing cognitive and functional profiles and assessing their change over time may inform differential diagnosis, particularly as cognitive decline is expected to be reflected in, or correlated with functional decline [53]. Domain-specific cognitive scores may be more useful than composite total scores in identifying impairment and decline in cognitive function related to regional brain function. Measuring domains such as attention, processing speed and executive function may increase the sensitivity of detecting disease progression and therapeutic effects, particularly in mild-to-moderate AD patients whose memory decline may be too slow to detect drug effects during a typical clinical trial. Further exploration of patterns of impairment may also have potential to be predictive of those at greatest risk of disease progression or who may benefit most from treatment. References [1] Levey, A., Lah, J., Goldstein, F., Steenland, K. and Bliwise, D. (2006) Mild Cognitive Impairment: An Opportunity to Identify Patients at High Risk for Progression to Alzheimer’s Disease. Clinical Therapeutics, 28, 991-1001. http://dx.doi.org/10.1016/j.clinthera.2006.07.006 [2] Petersen, R.C., Doody, R., Kurz, A., Mohs, R.C., Morris, J.C., Rabins, P.V., et al. (2001) Current Concepts in Mild Cognitive Impairment. JAMA Neurology, 58, 1985-1992. http://dx.doi.org/10.1001/archneur.58.12.1985 [3] Artero, S., Petersen, R., Touchon, J. and Ritchie, K. (2006) Revised Criteria for Mild Cognitive Impairment: Validation within a Longitudinal Population Study. Dementia and Geriatric Cognitive Disorders, 22, 465-470. http://dx.doi.org/10.1159/000096287 [4] Dubois, B., Feldman, H.H., Jacova, C., Dekosky, S.T., Barberger-Gateau, P., Cummings, J., et al. (2007) Research Criteria for the Diagnosis of Alzheimer’s Disease: Revising the NINCDS-ADRDA Criteria. Lancet Neurology, 6, 734- 746. [5] Birks, J. (2006) Cholinesterase Inhibitors for Alzheimer’s Disease. The Cochrane Database of Systematic Reviews, Article ID: CD005593. [6] Markwick, A., Zamboni, G. and de Jager, C.A. (2012) Profiles of Cognitive Subtest Impairment in the Montreal Cognitive Assessment (MoCA) in a Research Cohort with Normal Mini-Mental State Examination (MMSE) Scores. Journal of Clinical and Experimental Neuropsychology, 34, 750-757. http://dx.doi.org/10.1080/13803395.2012.672966 [7] Bondi, M.W., Jak, A.J., Delano-Wood, L., Jacobson, M.W., Delis, D.C. and Salmon, D.P. (2008) Neuropsychological Contributions to the Early Identification of Alzheimer’s Disease. Neuropsychology Review, 18, 73-90. http://dx.doi.org/10.1007/s11065-008-9054-1 [8] Karantzoulis, S. and Galvin, J.E. (2011) Distinguishing Alzheimer’s Disease from Other Major Forms of Dementia. Expert Review of Neurotherapeutics, 11, 1579-1591. http://dx.doi.org/10.1586/ern.11.155 [9] Nordlund, A., Rolstad, S., Hellstrom, P., Sjogren, M., Hansen, S. and Wallin, A. (2005) The Goteborg MCI Study: Mild Cognitive Impairment Is a Heterogeneous Condition. Journal of Neurology, Neurosurgery and Psychiatry, 76, 1485-1490. http://dx.doi.org/10.1136/jnnp.2004.050385 [10] Caine, D. and Hodges, J.R. (2001) Heterogeneity of Semantic and Visuospatial Deficits in Early Alzheimer’s Disease. Neuropsychology, 15, 155-164. http://dx.doi.org/10.1037/0894-4105.15.2.155 [11] Carter, S.F., Caine, D., Burns, A., Herholz, K. and Ralph, M.A.L. (2012) Staging of the Cognitive Decline in Alzheimer’s Disease: Insights from a Detailed Neuropsychological Investigation of Mild Cognitive Impairment and Mild Alzheimer’s Disease. International Journal of Geriatric Psychiatry, 27, 423-432. M. Marsico et al. 179 [12] Perry, R.J. and Hodges, J.R. (1999) Attention and Executive Deficits in Alzheimer’s Disease: A Critical Review. Brain, 122, 383-404. http://dx.doi.org/10.1093/brain/122.3.383 [13] Farias, S.T., Mungas, D., Reed, B.R., Harvey, D., Cahn-Weiner, D. and DeCarli, C. (2006) MCI Is Associated with Deficits in Everyday Functioning. Alzheimer Disease & Associated Disorders, 20, 217-223. http://dx.doi.org/10.1097/01.wad.0000213849.51495.d9 [14] Perneczky, R., Pohl, C., Sorg, C., Hartmann, J., Tosic, N., Grimmer, T., Heitele, S. and Kurz, A. (2006) Impairment of Activities of Daily Living Requiring Memory or Complex Reasoning as Part of the MCI Syndrome. International Journal of Geriatric Psychiatry, 21, 158-162. http://dx.doi.org/10.1002/gps.1444 [15] Reppermund, S., Brodaty, H., Crawford, J.D., Kochan, N.A., Draper, B., Slavin, M.J., et al. (2013) Impairment in Instrumental Activities of Daily Living with High Cognitive Demand Is an Early Marker of Mild Cognitive Impairment: The Sydney Memory and Ageing Study. Psychological Medicine, 43, 2437-2445. [16] Goldberg, T.E., Koppel, J., Keehlisen, L., Christen, E., Dreses-Werringloer, U., Conejero-Goldberg, C., Gordon, M.L. and Davies, P. (2010) Performance-Based Measures of Everyday Function in Mild Cognitive Impairment. American Journal of Psychiatry, 167, 845-853. http://dx.doi.org/10.1176/appi.ajp.2010.09050692 [17] Bangen, K.J., Jak, A.J., Schiehser, D.M., Delano-Wood, L., Tuminello, E., Han, S.D., et al. (2010) Complex Activities of Daily Living Vary by Mild Cognitive Impairment Subtype. Journal of the International Neuropsychological Society, 16, 630-639. http://dx.doi.org/10.1017/S1355617710000330 [18] Allaire, J.C., Gamaldo, A., Ayotte, B.J., Sims, R. and Whitfield, K. (2009) Mild Cognitive Impairment and Objective Instrumental Everyday Functioning: The Everyday Cognition Battery Memory Test. Journal of the American Geriatrics Society, 57, 120-125. http://dx.doi.org/10.1111/j.1532-5415.2008.02054.x [19] Mioshi, E., Kipps, C.M., Dawson, K., Mitchell, J., Graham, A. and Hodges, J.R. (2007) Activities of Daily Living in Frontotemporal Dementia and Alzheimer Disease. Neurology, 68, 2077-2084. http://dx.doi.org/10.1212/01.wnl.0000264897.13722.53 [20] Dangour, A.D., Allen, E., Richards, M., Whitehouse, P. and Uauy, R. (2010) Design Considerations in Long-Term Intervention Studies for the Prevention of Cognitive Decline or Dementia. Nutrition Reviews, 68, S16-S21. http://dx.doi.org/10.1111/j.1753-4887.2010.00330.x [21] de Jager, C.A., Hogervorst, E., Combrinck, M. and Budge, M.M. (2003) Sensitivity and Specificity of Neuropsychological Tests for Mild Cognitive Impairment, Vascular Cognitive Impairment and Alzheimer’s Disease. Psychological Medicine, 33, 1039-1050. http://dx.doi.org/10.1017/S0033291703008031 [22] de Jager, C.A. and Budge, M.M. (2005) Stability and Predictability of the Classification of Mild Cognitive Impairment as Assessed by Episodic Memory Test Performance over Time. Neurocase, 11, 72-79. http://dx.doi.org/10.1080/13554790490896820 [23] Hogervorst, E., Combrinck, M., Lapuerta, P., Rue, J., Swales, K. and Budge, M. (2002) The Hopkins Verbal Learning Test and Screening for Dementia. Dementia and Geriatric Cognitive Disorders, 13, 13-20. http://dx.doi.org/10.1159/000048628 [24] Kramer, J.H., Jurik, J., Sha, S.J., Rankin, K.P., Rosen, H.J., Johnson, J.K. and Miller, B.L. (2003) Distinctive Neuropsychological Patterns in Frontotemporal Dementia, Semantic Dementia, and Alzheimer Disease. Cognitive & Behavioral Neurology, 16, 211-218. http://dx.doi.org/10.1097/00146965-200312000-00002 [25] Libon, D.J., Massimo, L., Moore, P., Coslett, H.B., Chatterjee, A., Aguirre, G.K., et al. (2007) Screening for Frontotemporal Dementias and Alzheimer’s Disease with the Philadelphia Brief Assessment of Cognition: A Preliminary Analysis. Dementia and Geriatric Cognitive Disorders, 24, 441-447. http://dx.doi.org/10.1159/000110577 [26] Pendlebury, S.T., Markwick, A., de Jager, C.A., Zamboni, G., Wilcock, G.K. and Rothwell, P.M. (2012) Differences in Cognitive Profile between TIA, Stroke and Elderly Memory Research Subjects: A Comparison of the MMSE and MoCA. Cerebrovascular Diseases, 34, 48-54. http://dx.doi.org/10.1159/000338905 [27] Perry, R.J. and Hodges, J.R. (2000) Differentiating Frontal and Temporal Variant Frontotemporal Dementia from Alzheimer’s Disease. Neurology, 54, 2277-2284. http://dx.doi.org/10.1212/WNL.54.12.2277 [28] de Jager, C.A., Honey, T.E., Birks, J. and Wilcock, G.K. (2010) Retrospective Evaluation of Revised Criteria for the Diagnosis of Alzheimer’s Disease Using a Cohort with Post-Mortem Diagnosis. International Journal of Geriatric Psychiatry, 25, 988-997. http://dx.doi.org/10.1002/gps.2448 [29] Huppert, F.A., Brayne, C., Gill, C., Paykel, E.S. and Beardsall, L. (1995) CAMCOG—A Concise Neuropsychological Test to Assist Dementia Diagnosis: Socio-Demographic Determinants in an Elderly Population Sample. British Journal of Clinical Psychology, 34, 529-541. http://dx.doi.org/10.1111/j.2044-8260.1995.tb01487.x [30] Roth, M., Tym, E., Mountjoy, C.Q., Huppert, F.A., Hendrie, H., Verma, S. and Goddard, R. (1986) CAMDEX. A Standardised Instrument for the Diagnosis of Mental Disorder in the Elderly with Special Reference to the Early Detection of Dementia. British Journal of Psychiatry, 149, 698-709. http://dx.doi.org/10.1192/bjp.149.6.698 M. Marsico et al. 180 [31] Folstein, M.F., Folstein, S.E. and McHugh, P.R. (1975) “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. Journal of Psychiatric Research, 12, 189-198. http://dx.doi.org/10.1016/0022-3956(75)90026-6 [32] Brandt, J., Spencer, M. and Folstein, M. (1988) The Telephone Interview for Cognitive Status. Neuropsychiatry, Neuropsychology, & Behavioral Neurology, 1, 111-117. [33] Morris, J.C. (1997) Clinical Dementia Rating: A Reliable and Valid Diagnostic and Staging Measure for Dementia of the Alzheimer Type. International Psychogeriatrics, 9, 173-176. http://dx.doi.org/10.1017/S1041610297004870 [34] Yesavage, J.A., Brink, T.L., Rose, T.L., Lum, O., Huang, V., Adey, M. and Otto Leirer, V. (1982) Development and Validation of a Geriatric Depression Screening Scale: A Preliminary Report. Journal of Psychiatric Research, 17, 37-49. http://dx.doi.org/10.1016/0022-3956(82)90033-4 [35] Ferris, S.H., Lucca, U., Mohs, R., Dubois, B., Wesnes, K., Erzigkeit, H., et al. (1997) Objective Psychometric Tests in Clinical Trials of Dementia Drugs. Position Paper from the International Working Group on Harmonization of Dementia Drug Guidelines. Alzheimer Disease and Associated Disorders, 11, 34-38. [36] Rosen, W.G., Mohs, R.C. and Davis, K.L. (1984) A New Rating Scale for Alzheimer’s Disease. American Journal of Psychiatry, 141, 1356-1364. [37] Cambridge Cognition Ltd. (2013) Cambridge Cognition—The Home of CANTAB. CANTAB.com [Internet]. Cambridge Cognition Ltd., Cambridge, c2013. http://www.cantab.com/ [38] Galasko, D., Bennett, D., Sano, M., Ernesto, C., Thomas, R., Grundman, M. and Ferris, S. (1997) An Inventory to Assess Activities of Daily Living for Clinical Trials in Alzheimer’s Disease. Alzheimer Disease & Associated Disorders, 11, S33-S39. http://dx.doi.org/10.1097/00002093-199700112-00005 [39] Sunderland, A., Harris, J. and Baddeley, A. (1983) Do Laboratory Tests Predict Everyday Memory? A Neuropsychological Study. Journal of Verbal Learning and Verbal Behavior, 22, 341-357. http://dx.doi.org/10.1016/S0022-5371(83)90229-3 [40] Graham, D., Cully, J.A., Snow, A.L., Massman, P. and Doody, R. (2004) The Alzheimer’s Disease Assessment ScaleCognitive Subscale: Normative Data for Older Adults Controls. Alzheimer Disease and Associated Disorders, 18, 236- 240. [41] Grundman, M., Petersen, R.C., Ferris, S.H., Thomas, R.G., Aisen, P.S., Bennett, D.A., et al. (2004) Mild Cognitive Impairment Can Be Distinguished from Alzheimer Disease and Normal Aging for Clinical Trials. JAMA Neurology, 61, 59-66. http://dx.doi.org/10.1001/archneur.61.1.59 [42] Royall, D.R., Cordes, J.A. and Polk, M. (1998) CLOX: An Executive Clock Drawing Task. Journal of Neurology, Neurosurgery & Psychiatry, 64, 588-594. http://dx.doi.org/10.1136/jnnp.64.5.588 [43] Smith, A. (1968) The Symbol-Digit Modalities Test: A Neuropsychologic Test of Learning and Other Cerebral Disorders. In: Learning Disorders, Special Child Publications, Seattle, 83-91. [44] Robertson, I.H., Thames Valley Test Company (1994) The Test of Everyday Attention. Thames Valley Test Company. [45] Brandt, J., Aretouli, E., Neijstrom, M.S., Samek, J., Manning, K., Albert, M.S., et al. (2009) Selectivity of Executive Function Deficits in Mild Cognitive Impairment. Neuropsychology, 23, 607-618. http://dx.doi.org/10.1037/a0015851 [46] Papp, K.V., Snyder, P.J., Maruff, P., Bartkowiak, J. and Pietrzak, R.H. (2011) Detecting Subtle Changes in Visuospatial Executive Function and Learning in the Amnestic Variant of Mild Cognitive Impairment. PLoS ONE, 6, e21688. http://dx.doi.org/10.1371/journal.pone.0021688 [47] Voss, S. and Bullock, R. (2004) Executive Function: The Core Feature of Dementia? Dementia and Geriatric Cognitive Disorders, 18, 207-216. http://dx.doi.org/10.1159/000079202 [48] Brandt, J. (1991) The Hopkins Verbal Learning Test: Development of a New Memory Test with Six Equivalent Forms. Clinical Neuropsychologist, 5, 125-142. http://dx.doi.org/10.1080/13854049108403297 [49] Cano, S.J., Posner, H.B., Moline, M.L., Hurt, S.W., Swartz, J., Hsu, T. and Hobart, J.C. (2010) The ADAS-Cog in Alzheimer’s Disease Clinical Trials: Psychometric Evaluation of the Sum and Its Parts. Journal of Neurology, Neurosurgery & Psychiatry, 81, 1363-1368. http://dx.doi.org/10.1136/jnnp.2009.204008 [50] Petersen, R.C., Smith, G.E., Waring, S.C., Ivnik, R.J., Tangalos, E.G. and Kokmen, E. (1999) Mild Cognitive Impairment: Clinical Characterization and Outcome. JAMA Neurology, 56, 303-308. http://dx.doi.org/10.1001/archneur.56.3.303 [51] Winblad, B., Palmer, K., Kivipelto, M., Jelic, V., Fratiglioni, L., Wahlund, L.O., et al. (2004) Mild Cognitive Impairment—Beyond Controversies, towards a Consensus: Report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine, 256, 240-246. http://dx.doi.org/10.1111/j.1365-2796.2004.01380.x [52] Marshall, D.A., Rentz, D.M., Frey, M.T., Locascio, J.J., Johnson, K.A. and Sperling, R.A., the Alzheimer’s Disease Neuroimaging Initiative (2011) Executive Function and Instrumental Activities of Daily Living in Mild Cognitive Im- M. Marsico et al. 181 pairment and Alzheimer’s Disease. Alzheimer’s & Dementia, 7, 300-308. http://dx.doi.org/10.1016/j.jalz.2010.04.005 [53] Perry, R.J. and Hodges, J.R. (2000) Relationship between Functional and Neuropsychological Performance in Early Alzheimer Disease. Alzheimer Disease & Associated Disorders, 14, 1-10. http://dx.doi.org/10.1097/00002093-200001000-00001 [54] Cornish, I.M. (2000) Factor Structure of the Everyday Memory Questionnaire. British Journal of Psychology, 91, 427- 438. http://dx.doi.org/10.1348/000712600161916 [55] Salthouse, T.A. and Babcock, R.L. (1991) Decomposing Adult Age Differences in Working Memory. Developmental Psychology, 27, 763-776. http://dx.doi.org/10.1037/0012-1649.27.5.763 [56] Anderson, E., De Jager, C. and Iversen, S. (2006) The Placing Test: Preliminary Investigations of a Quick and Simple Memory Test Designed to Be Sensitive to Pre-Dementia Alzheimer’s Disease but Not to Normal Ageing. Journal of Clinical and Experimental Neuropsychology, 28, 843-858. http://dx.doi.org/10.1080/13803390591001016 [57] McKenna, P. and Warrington, E.K. (1980) Testing for Nominal Dysphasia. Journal of Neurology, Neurosurgery & Psychiatry, 43, 781-788. http://dx.doi.org/10.1136/jnnp.43.9.781